The kinetics of epoxidation of trimethylolpropane ester
Abstract
Kinetics pertaining epoxidation reaction of a palm oil-based synthetic lubricant trimethylolpropane (TMP) ester were investigated. The epoxidation reaction of TMP ester was carried out utilizing peracetic acid generated by an in situ technique. The analysis of the reaction kinetics was performed within the low temperature (30, 50, and 60°C) and high temperature (70, 80, and 90°C) regions, owing to the nature of the reactions. The maximum conversion of the unsaturated carbon to oxirane ring was achieved in 1 h at high temperature region, while epoxidation of TMP esters took more than 4 h to reach the maximum conversion at the low temperature region. From the experimental data, the kinetics of epoxidation of TMP esters fitted well with both the second-order and pseudo first-order models. The rate constants for pseudo first-order model increased from 0.0009 to 0.0055 by increasing temperature at the low temperature region, and from 0.0129 to 0.0209 within the high temperature region. The values of activation energies at low temperature and high temperature regions were found to be 69.4 and 53.3 kJ/mol, respectively.
Abbreviations:
AA, acetic acid; PA, peracetic acid; PME, palm oil methyl ester; RCO, relative conversion to oxirane; TMP, trimethylolpropane; VI, viscosity index
Introduction
Trimethylolpropane (TMP) triester (TMP trioleate) has been well accepted as the base stock in lubricant formulation 1. Oxidation is a major concern that limits the use of vegetable oils as lubricating fluids. It leads to polymerization and degradation causing the decrease in lubrication functionality, breakdown of volatile and corrosive products, and diminishing of the structure and properties of the lubricants 2. Therefore, improving the oxidative stability of biolubricants is important in maintaining their sustainability and performance as lubricants.
One of the means to improve oxidative stability of a biolubricant is to decrease the level of unsaturation in the vegetable oil; unsaturated CC bonds provide a vulnerable site for the oxidation reaction. Such elimination of double bonds can be achieved by epoxidation of oils during which the double bond is converted into an oxirane ring aided by a suitable oxidizing agent. On the industrial scale, the epoxidation process is generally carried out by peracetic (PA) or performic acids, which are executed in situ. In situ epoxidation technique has already been attempted by various researchers employing different feedstocks i.e., canola oil 3, cottonseed oil 4, madhuca and neem oil 5, rubber seed oil 6, sunflower oil 7, mahua oil 8, and soybean oil 9.
The in situ peracid process was used for the epoxidation of a palm oil-based synthetic lubricant, chemically known as TMP ester. In situ peracid process was selected mainly due to the safety concerns, since the concentrated peracid is unstable and explosive if generated externally 10. Epoxidation of TMP ester was performed utilizing a homogeneous catalyst; reactions are presented in Scheme 1. The in situ epoxidation reaction takes place in two steps; step one ensures the formation of PA, and the reaction of PA with the ethylenic unsaturation takes place to form the oxirane ring in the second step.
In situ PA process in epoxidation reaction.
In the previous studies, the researchers focused on epoxidation of vegetable oils and none so far consider the improvement on the oxidative stability of TMP triester. This is because most of the commercially available TMP esters are in the form of TMP trioleate. Study on epoxidation of TMP trioleate is not done because it is considered as oxidatively stable oil. However, compared to saturated fatty acids such as palmitic, oleic acid contains a single double bond of which is still highly susceptible to oxidation degradation. In this study, palm-based TMP triester was synthesized and later epoxidized to examine the effect of epoxidation on the oxidative stability of palm-based TPM ester. The final consideration of this work was to calculate the activation energy of epoxidized TMP esters and its comparison with the activation energies reported for other vegetable oils.
Materials and methods
Materials
Palm oil-based synthetic lubricant used in the experiment was TMP ester (TMPe), which was synthesized in the laboratory by transesterification of palm oil methyl ester (PME) and TMP with sodium methoxide catalyst 11. Fatty acid composition of PME is shown in Table 1. The synthesized TMP ester consisted of 98% w/w triesters and served as a starting material for the epoxidation reaction. The composition of TMP ester was analyzed using GC following the method described by Yunus et al. 12. Other chemicals used in the epoxidation reaction were glacial acetic acid (AA, 99.7% v/v) (Rankem, India), heptane (Merck, Germany), sulfuric acid (96.5% v/v) (Rdh, Germany), and hydrogen peroxide (35% v/v) (Merck, Germany).
| Fatty acid | PME (%) |
|---|---|
| Capric C10:0 | – |
| Lauric C12:0 | 0.9 |
| Myristic C14:0 | 1.5 |
| Palmitic C16:0 | 41.5 |
| Stearic C18:0 | 2.7 |
| Oleic C18:1 | 40.6 |
| Linoleic C18:2 | 11.9 |
| Linolenic C18:3 | 0.3 |
| Others | 0.2 |
Kinetic study for epoxidation reactions of TMP ester
TMP ester was used as a starting material in the epoxidation reaction where the reaction was carried out based on the procedures described by Kirk and Othmer 13. The required amount of TMP ester and a solvent (heptanes) were added into the three necked reaction flask (500 mL) connected to a reflux condenser. PA was prepared in situ by reacting 0.5 mole of AA with 7.5 mole of hydrogen peroxide in the presence of 2% v/v of concentrated sulfuric acid catalyst. The PA mixture was then added into a mixture of TMPe and heptane. In order to minimize oxirane cleavage, approximately 20% v/v heptanes was used as a solvent. The mixture was heated up to the desired temperature with continuous stirring. The reactions were performed at six different temperatures, namely 30, 50, 60, 70, 80, 90 ± 1°C for 10 h. Since the reaction is exothermic, the temperature of reaction was carefully controlled through a sufficient supply of cooling water. Samples were taken after particular time intervals for oxirane and iodine value analyses. The percentage of oxirane oxygen analysis was done using the direct method Cd 9–57 by American Oil Chemists' Society (AOCS) 14. While the iodine value was analyzed by Wij's method based on the PORIM Test Method 15.
Analysis
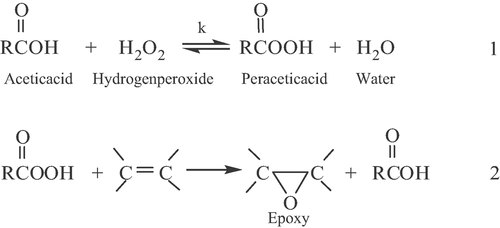
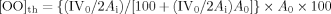 ((1))
((1))The values of Ai = 126.9 and A0 = 16.0 represent the atomic numbers for iodine and oxygen, respectively. The initial iodine value (IV0) of the oil sample was determined to be 66.4 from iodine value analysis.
Results and discussion
The kinetics study on epoxidation of TMP esters was performed at six different temperatures, i.e., 30, 50, 60, 70, 80, and 90°C for 10 h. The effect of the change in temperature and time on the percentage of oxirane oxygen is shown in Fig. 1. The reaction conversion was determined based on the epoxidation of the double bond, present in the fatty acids chain of TMP esters, into oxirane oxygen. At higher temperatures, the rate of reaction was considerably fast and the reaction took less time to reach its completion. At lower temperatures, the maximum conversion of oxirane oxygen took place in more than 4 h, while at higher temperatures the maximum conversion of oxirane oxygen was achieved in less than 1 h. Since the rate of reaction for the low temperature region was considerably less as compared to the high temperature region, so the analysis of the reaction kinetics was carried out by distributing temperature into two separate regions, namely low temperature (30, 50, and 60°C) and high temperature (70, 80, and 90°C) regions. Stamenković et al. 17 discovered that reaction mechanism of sunflower oil methanolysis can be divided into two regions namely the initial mass transfer-controlled region and the kinetically controlled region. They used different rate equations for the two regions due to the different mechanisms involved.
Effect of temperature and time on percentage oxirane oxygen.
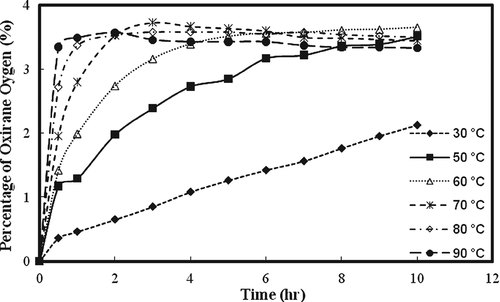
The effect of temperature and time on the RCO under low temperature and high temperature regions is presented in Fig. 2. The figure indicates the proportional increase in percentage RCO with the increase in temperature and reaction time. At low temperature the rate of formation of oxirane ring was typically slow. The reaction took long time to complete which might be contributed toward the degradation of oxirane; however, there was no indication for the degradation of oxirane ring to the hydroxyl group in Fig. 2(a). The highest conversion to oxirane was 84.3% which occurred at 60°C after 4 h reaction time at low temperature region. At high temperature region, the highest conversion to oxirane was achieved at 90°C within 1 h.
Effects of temperature and time on RCO at: (a) low temperature region (b) high temperature region.
Biolubricant properties
The properties of lubricants, particularly oxidative stability, pour point, and viscosity index (VI) are influenced by the structure of molecules 18. When structural modification is done through epoxidation, a prominent effect on the properties can be observed. The effect of epoxidation of TMP esters on its properties has been depicted in Table 2. The epoxidation has improved the oxidative stability of the original TMP ester at the expense of pour point and VI.
| Samples | Pour point (°C) | Kinematic viscosity, cSt @40°C | Kinematic viscosity, cSt @100°C | Viscosity index (VI) | TAN (after 500 h) | Iodine value (mg I2/g oil) | Oxidative stability (TOST) (acid value after 500 h) |
|---|---|---|---|---|---|---|---|
| Epoxidized TMP esters | 1 | 55.13 | 11.04 | 197 | 6.4 | 2 | 2.9 |
| TMP esters | −4 | 40.42 | 9.29 | 223 | 2.9 | 54 | 6.4 |
Order of kinetic models
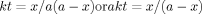 ((2))
((2))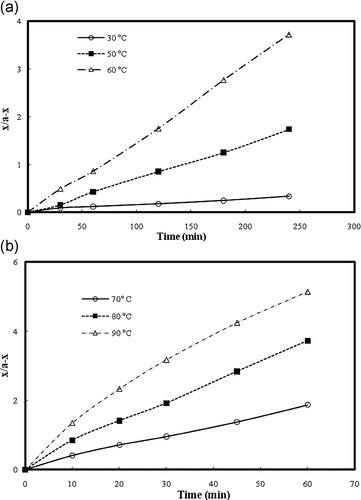
Second order kinetics for epoxidation reaction of TMP esters at: (a) low temperature region (b) high temperature region.
The second order rate constants were obtained from the slope and intercept of the plot; the results are tabulated in Table 3. High dependency of reaction rate constants on temperature is evident. This might be due to the increase in speed and collision of particles with the increase in temperature since the reaction takes place when the reactant particles collide. Therefore, rate constants for high temperature region (70, 80, 90°C) were expectedly higher than at lower temperature region (30, 50, 60°C). The reaction rate was around four times faster at higher temperature region than at lower temperature region and it took 60 min for reaction completion at higher temperatures opposed to 240 min at lower temperatures.
| Temperature (°C) | Reaction time (min) | Rate constant | R2 |
|---|---|---|---|
| Low temperature region | |||
| 30 | 240 | 0.0013 | 0.9747 |
| 50 | 240 | 0.0073 | 0.9983 |
| 60 | 240 | 0.0155 | 0.9988 |
| High temperature region | |||
| 70 | 60 | 0.03 | 0.9956 |
| 80 | 60 | 0.0603 | 0.9958 |
| 90 | 60 | 0.0837 | 0.9775 |
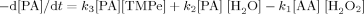 ((3))
((3))- (1)
Hydrogen peroxide (H2O2) is used in excess. The stoichiometric ratio of H2O2 to AA is 1:1. However, to ensure that H2O2 was in excess, the ratio was increased to 15:1 (H2O2/AA). (See the methodology section).
- (2)
[PA] is constant during the reaction, so d[PA]/dt = 0. The concentration of PA can be assumed constant because the amount of PA used for the epoxidation of double bond in TMP ester (Reaction 2) is negligible due to small number of double bond available in palm oil TMP ester 20.
- (3)
Backward reaction is negligible, so k2[PA] [H2O] = 0. The use of large excess of H2O2 (15:1) justifies the assumption of negligible backward reaction with respect to the forward reaction (Reaction 1).
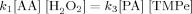 ((4))
((4))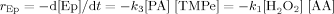 ((5))
((5))Since H2O2 is in excess, so: [H2O2] = [H2O2]0 − [H2O2]consumed and [Ep] = [H2O2]consumed
 ((6))
((6)) ((7))
((7))A graph was plotted for ln{[H2O2]0 − [Ep]} versus time, shown in Fig. 4, where the subscript “o” denotes the initial concentration while Ep denotes the epoxidized TMP. The graph disclosed that the pseudo first order model also fits well for all the reaction temperatures. The correlation coefficients for epoxidized TMP esters at low temperatures (30, 50, and 60°C) were 0.9957, 0.9999, and 0.9863 while at high temperatures (70, 80, and 90°C) were 0.9996, 0.9873, and 0.9416, respectively.
Pseudo first order kinetics for epoxidation reaction of TMP esters at: (a) low temperature region (b) high temperature region.
The rate constants following pseudo-first order reaction kinetics for epoxidation of TMP esters were also calculated and tabulated in Table 4. The values of rate constants increased from 0.0009 to 0.0055 when the temperature was increased inside low temperature region from 30 to 60°C. Similarly, at high temperature region the values increased from 0.0129 to 0.0209 with the increase in temperature from 70 to 90°C. Higher temperatures let reactant molecules to possess energy in excess to the reaction energy barrier and hence the rate of reaction increases 21.
| Temperature (°C) | Reaction time (h) | Rate constant | R2 |
|---|---|---|---|
| Low temperature region | |||
| 30 | 240 | 0.0009 | 0.9957 |
| 50 | 240 | 0.0033 | 0.9999 |
| 60 | 240 | 0.0055 | 0.9863 |
| High temperature region | |||
| 70 | 60 | 0.0129 | 0.9996 |
| 80 | 60 | 0.0193 | 0.9873 |
| 90 | 60 | 0.0209 | 0.9416 |
By comparing the regression values obtained from the second order and pseudo-first order models (Tables 3 and 4), the second order model appears more suitable for both low and high temperature regions than pseudo-first order model due to higher R2 values. Nevertheless, pseudo-first order model is simpler to use as the plot only requires the data for initial hydrogen peroxide concentration, AAs concentration and the final oxirane ring content.
Activation energy (Ea)
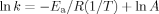 ((8))
((8))In this relation, A is assigned as the frequency factor and E is called the activation energy of the reaction. A plot of ln k versus 1/T is the Arrhenius plot and it generates a slope equal to Ea/R.
The Arrhenius plot for the epoxidation of TMP esters at low temperature and high temperature regions is demonstrated in Fig. 5. The rate constants were obtained from the second order kinetics model. The values of R2 for the low temperature and high temperature plots are 0.9999 and 0.9684, respectively. The slope of the Arrhenius plot was availed to determine the activation energies at low temperature and high temperature regions, and the values were found to be 69.4 and 53.3 kJ/mol, respectively. The higher activation energy at low temperature in contrast to high temperature elucidates that the reactant molecules need more energy for overcoming the energy barrier in order to react with each other at low temperature. These values were comparable with the values of activation energies reported for epoxidation of: cottonseed oil, 48.67 kJ/mol 4; rubber seed oil, 65.31 kJ/mol 6; mahua oil, 60.32 kJ/mol 8; methyl esters of palm olein, 76.12 kJ/mol 22; soybean oil, 43.11 kJ/mol; corn oil, 74.22 kJ/mol; and sunflower oil, 85.21 kJ/mol 20.
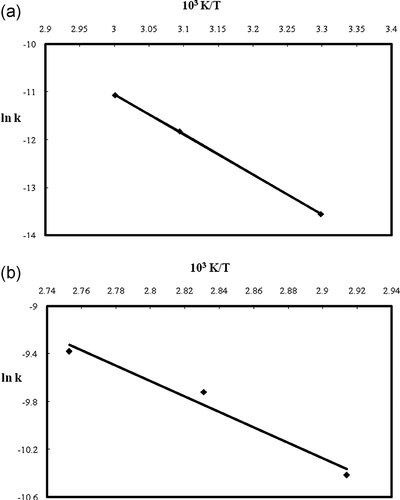
Arrhenius plot for the temperature dependency of the reaction rate constant in second-order reaction at (a) low temperature region (b) high temperature region.
The difference in the values of activation energy for various oils can probably be due to the variation in their fatty acid compositions. Oils with high content of polyunsaturated fatty acid, like linolenic acid, would be easier to epoxidize by requiring less activation energy 22. In addition, the reactants necessitating less activation energy can initiate a reaction which is less sensitive toward temperature changes.
Conclusions
The kinetics study on the epoxidation of TMP esters by PA was carried out to determine the kinetics parameters. PA was formed in situ in presence of sulfuric acid as a catalyst. The results from the present study indicated that the epoxidation of TMP esters by PA generated in situ can be carried out at higher temperatures to achieve the maximum conversion to oxirane ring. For low temperature region, reaction took more time to complete the conversion as compared to higher temperature region. Higher temperature was found to be more effective due to exothermic nature of reaction. The kinetic parameters of epoxidation obtain from the study indicate that an increase in temperature would increase the rate of epoxide formation.
Acknowledgements
This research was supported by Department of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia and FRGS grant no. 5523430.
The authors have declared no conflict of interest.




