Glucocorticoid-induced osteonecrosis in systemic lupus erythematosus patients
Abstract
Osteonecrosis (ON) is a complex and multifactorial complication of systemic lupus erythematosus (SLE). ON is a devastating condition that causes severe pain and compromises the quality of life. The prevalence of ON in SLE patients is variable, ranging from 1.7% to 52%. However, the pathophysiology and risk factors for ON in patients with SLE have not yet been fully determined. Several mechanisms for SLE patients’ propensity to develop ON have been proposed. Glucocorticoid is a widely used therapeutic option for SLE patients and high-dose glucocorticoid therapy in SLE patients is strongly associated with the development of ON. Although the hips and knees are the most commonly affected areas, it may be present at multiple anatomical locations. Clinically, ON often remains undetected until patients feel discomfort and pain at specific sites at which point the process of bone death is already advanced. However, strategies for prevention and options for treatment are limited. Here, we review the epidemiology, risk factors, diagnosis, and treatment options for glucocorticoid-induced ON, with a specific focus on patients with SLE.
1 INTRODUCTION
Osteonecrosis (ON) (also known as avascular necrosis (AVN), atraumatic necrosis, aseptic necrosis, or ischemic necrosis) is a pathologic process of bone cell death that typically affects the middle-aged population.1-3 ON is a debilitating disease, causing severe pain and compromised quality of life; ON can occur in several circumstances. Trauma is the most common cause. Atraumatic causes of ON are alcohol and glucocorticoid use, hypercholesterolemia, sickle cell anemia, and autoimmune diseases.4-10 The use of glucocorticoids is one of the most common causes of nontraumatic ON.9, 11-14
Osteonecrosis is a well-known complication of adult and juvenile autoimmune diseases15, 16 and occurs more frequently in patients with systemic lupus erythematosus (SLE) than in any other rheumatic diseases requiring administration of glucocorticoid.17, 18 The prevalence of ON in patients with SLE varies between 1.7% and 52%. However, the etiology of ON in SLE patients is incompletely understood. Among several risk factors, glucocorticoid use is strongly associated with the development of ON in patients with SLE.19-23 At present, strategies for the prevention and treatment of glucocorticoid-induced ON are limited, with no effective therapy that can reverse the conditions, primarily because the pathogenesis of ON is poorly understood. In this review, we examine recent evidence of the pathogenesis, risk factors, diagnosis, and treatment options of glucocorticoid-induced ON in SLE patients.
2 THE ACTION OF GLUCOCORTICOIDS
Corticosteroids, which are endogenous hormones derived from cholesterol in the adrenal glands, have been used exogenously as anti-inflammatory and immunosuppressive agents for decades.24 Typically, the human body produces corticosteroids in response to many different stimuli. They are metabolized into glucocorticoids and mineralocorticoids and control many of the body's regulatory processes. Mineralocorticoids such as aldosterone regulate the electrolyte and volume status of the human body. Glucocorticoids, the main actor being cortisol, are endogenously released due to stresses on the body.25, 26
HIGHLIGHTS
-
The use of glucocorticoid treatments is one of the major risk factors for osteonecrosis in SLE patients.
-
The pathogenesis of glucocorticoid-induced osteonecrosis in SLE patients remains unclear.
-
Glucocorticoid-mediated changes including changes in angiogenesis, apoptosis of osteocyte, osteoblast, and endothelial cells, as well as adipogenesis and fat hypertrophy, may contribute to the onset of osteonecrosis in SLE patients.
The glucocorticoids from one of the most influential classes of modern medications. Their clinical application began in 1948 when Dr. Philip Showalter Hench used synthetic cortisone for the first time in a patient with rheumatoid arthritis.14 Due to their immunosuppressive and anti-inflammatory effects, glucocorticoids have been widely used to manage autoimmune conditions, inflammatory diseases, allergies, and hematological disorders. These disorders include multiple sclerosis, glomerular disease, Sjögren's disease, sarcoidosis, Graves’ disease, SLE, and rheumatoid arthritis. Glucocorticoids are also used for the local symptomatic treatment of osteoarthritis and tenosynovitis..27, 28 However, various side effects of long-term treatment, such as ON and osteoporosis, have been noted.29-31
Glucocorticoids robustly impact numerous tissues and cell lineages. They induce apoptosis of immune cells such as T cells, basophils, and eosinophils by modifying cytokine release profiles and dampening the immune response and are used to trigger apoptosis in tumor cells as a treatment for multiple myeloma, Hodgkin's lymphoma, and chronic lymphoblastic leukemia.32 Glucocorticoids also lead to increased levels of vasoconstriction in peripheral tissues. In addition to the direct impact on immune cells, glucocorticoids lead to changes at the tissue level, leading to side effects such as osteoporosis, euphoria, psychosis, hyperglycemia, and osteonecrosis.33
3 GLUCOCORTICOID-INDUCED OSTEONECROSIS
3.1 Methods
A comprehensive and systematic search was performed using the MEDLINE/PubMed (U.S. National Library of Medicine, Bethesda, MD). The database was searched in November 2020 from its inception to 2020 for only articles written in or translated into English. A broad combination of Medical Subject Heading (MeSH) terms were used, including “(systemic lupus erythematosus OR SLE) AND reconstruction AND (osteonecrosis OR avascular osteonecrosis OR aseptic necrosis) AND (glucocorticoids OR corticosteroids).”
3.2 Epidemiology
Table 1 summarizes the frequency of ON and the risk factors for the development of ON in SLE patients reported by prospective, retrospective, and cohort studies. The association between SLE and ON was first reported in 1960.19 Studies have reported incidence rates for ON in SLE patients ranging from 1.7% to 52% (Table 1). This wide range reflects the use of different techniques to diagnose ON (from the less sensitive plain film radiograph to the fairly sensitive MRI), variations in glucocorticoid dosing, and different durations of follow-up. In addition, the wide variation in prevalence may result from a missed diagnosis of asymptomatic ON.34 Data from prospective studies are similarly variable.25, 51, 91, 93, 94, 103, 114, 117-119, 196, 199 In one study, the incidence of ON increased constantly during each of the first 5 years after a diagnosis of SLE. Only one (0.27%) of 365 SLE patients developed ON in the first year after diagnosis, compared to 0.88% and 3.3% during the second and fourth years after diagnosis.35 In another long-term cohort study, 50% of SLE-diagnosed patients developed ON within 2 years of diagnosis.36 In the largest cohort of SLE patients with symptomatic ON, 234 of 1729 (13.5%) patients with SLE had 581 sites of symptomatic ON. The hips and knees were the most common of these sites and 47% of the patients had multiple sites involved.37
| Clinical factors | ||||||
|---|---|---|---|---|---|---|
| Author | Year | Study design | No. of patients | Prevalence (%) | Related factors | Unrelated factors |
| Dogan et al40 | 2020 | Cross-sectional | 127 | 8.7% (11) | l, u, cc, ff, hh | e, g, o, p, s, w, x |
| Tsai et al.98 | 2020 | Retrospective | 1472 | 2.6% (39) | l, aa | a, l, w, z, ff, gg |
| Kwon HH et al.99 | 2018 | Observational | 1219 | 10.8% (132) | w, z | a, g, l, m, p, r, v, y |
| Ruiz-Arruza I et al.60 | 2018 | Observational | 287 | 2.4% (7) | Not applicable | Not applicable |
| Gladman DD et al.37 | 2018 | Prospective | 1729 | 13.5% (234) | c, t, u, ff | a, x |
| Chen HL et al.113 | 2018 | Prospective | 11288 | 3.9% (444) | u | Not applicable |
| Tse SM et al.46 | 2017 | Retrospective | 275 | 7.4% (55) | a, t, u, w, cc, dd, ff | e, f, g, h, I, j, n, m, o, p, gg |
| Sheane BJ et al.47 | 2017 | Prospective | 173 | 13.9% (24) | u | s, t |
| Kuroda et al.100 | 2015 | Prospective# | 78 | 26.9% (21) | ff | a, b, f, g, j, m, l, s, y |
| Faezi et al101 | 2015 | Retrospective case-control | oral (314) pulse (351) |
21% (66) 11% (39) |
a, f, g, m | e, i, l, m, n, p, q, s, hh, ff |
| Yang et al.16 | 2015 | Case-control | 617 | 6% (37) | l, m, u | a, e, q, s, v, z, aa |
| Gontero et al.102 | 2015 | Observational | 158 | 9.5% (15) | t, cc | a, e, g, i, k, l, m, n, o, q, r, s, cc, gg |
| Joo et al.48 | 2015 | Retrospective | 25,358 |
3.15-3.42% (8.4-9.8/1000) |
u, y, z, aa, bb, gg | a, b, l, hh |
| Lee et al.49 | 2014 | Retrospective | 1051 | 6.9% (73) | u, w, z, cc | a, b, d, e, f, g, h, i, l, m, n, p, q, r, gg, hh |
| Ruiz-Arruza I et al.115 | 2014 | Observational | 230 | 1.7% (4) | u | Not applicable |
| Kunyakham et al.103 | 2012 | Retrospective | 736 | 8.8% (65) | d, n | a, b, g, i, n, j, l, m, o, u, z, aa, dd, ff, gg |
| Sayarlioglu et al.50 | 2012 | Retrospective | 868 | 5.6% (49) | a, b, e, f, h, j, m, o, u, w, x, z | g, i, n, l, q, r, ee, ff |
| Nakamura et al.104 | 2010 | Prospective | 676 | 38.5% (260) | a | U |
| Al Saleh et al.66 | 2010 | A cross-sectional and retrospective case-control | 126 | 8.7% (11) | g, j, l, o, q, r, t, v, z, aa | e, f, i, n, ff, gg |
| Sekiya et al.65 | 2010 | Prospective# | 17 | 29.4% (5) | q, dd | a, b, n, r, u, y |
| Uea-areewongsa et al.105 | 2009 | Case-control | 186 | 22% (41) | l, aa | a, d, m, s, u, w, x, z, ff |
| Fialho et al.106 | 2007 | Prospective | 46 | 21.7% (10) | s | e, g, o, q, t, w, z, aa, cc, ff |
| Prasad et al.107 | 2007 | Case-control | 570 | 11.4% (65) | Not applicable | c, e, i, l, q, s, u, x, y, z, aa, ff, hh |
| Nagasawa et al.51 | 2005 | Prospective# | 45 | 44.4% (20) | u, y, ff | a, b, l, m, n, q, v |
| Oinuma et al.52 | 2001 | Prospective# | 72 | 44% (32) | u | a, b, s, y |
| Gladman et al.36 | 2001 | Case-control | 744 | 12.8% (95) | i, u, z, | a, b, c, d, e, l, m, o, q, s, aa, cc, ff |
| Gladman et al.53 | 2001 | Case-control 70 patients used |
744 | 12.8% (95) | i, u, w, x, z, cc | a, b, c, d, e, g, l, m, n, o, q, r, s, y, ff |
| Mok et al.67 | 2000 | Retrospective | 265 | 4.2% (11) | No association | a, b, c, d, u, w, q |
| Zonana-Nacach et al.54 | 2000 | Retrospective | 539 | 8.7% (47) | u | w, t, gg |
| Mok et al.42 | 1998 | Case-control | 320 | 12% (38) | m, q, u, w, z, aa, cc | a, b, d, e, f, l, m, n, o, q, r, s, x, y, gg |
| Cozen et al.39 | 1998 | Follow-up | 488 | 5% (26) | a, j, l, m, n, gg | c, e, g, i, q, r, u, y, aa |
| Mont et al.55 | 1997 | Cohort | 103 | 30% (31) | o, q, u, cc, ii | c, g, i, l, n, ee, gg |
| Arranow et al.41 | 1997 | Retrospective | 66 | 12% (8) | e, m, u | c, o, q, y |
| Rascu et al.108 | 1996 | Retrospective | 280 | 2.1% (6) | Not applicable | e, i, j, l, m, n, o, q, r, u, w, x, y |
| Migliaresi et al.56 | 1994 | Observational | 69 | 10.14% (7) | u | a, d, q, r, w, y |
| Nagasawa et al.34 | 1994 | Prospective | 23 | 48% (11) 10% (3); syntomic ON |
u | a, b, x |
| Asherson et al.64 | 1993 | Retrospective | 800 | 4.6% (37) | Not applicable | Not applicable |
| Massardo et al.109 | 1992 | Retrospective | 176 | 9.7% (17) | v, y, cc | a, b, e, i, j, l, m, n, o |
| Ono et al.57 | 1992 | Prospective | 62 | 14.5% (9) | f, k, l, n, u, ff | e, i, j, m, n, r |
| Weiner et al.58 | 1989 | Follow-up | 172 | 16.2% (28) | u | e, f, g, i, l, m, n, o, u, cc |
| Kalla et al.110 | 1986 | Retrospective* | 185 | 7% (13) | Not applicable | e, w, x, z, ff |
| Zizic et al.17 | 1985 | Prospective | 54 | 52% (28) | u, x | a, b, c, d, e, h, i, l, m, n, q, r, v, cc, ff |
| Klippel et al.43 | 1979 | Retrospective | 375 | 8.3% (31) | u | a, b, e, l, m |
| Albeles et al.35 | 1978 | Follow-up* | 365 | 4.7% (17) | v | a, b, s, u, x |
| Dimant et al.111 | 1978 | Retrospective case-control | 234 | 9% (22) | Not applicable | a, d, l, o, s, u, w, x |
| Smith et al.112 | 1976 | Retrospective case-contro | 99 | 7% (7) | Not applicable | a, e, f, i, j, l, m, n, r, u, w, gg |
| Bergstein et al.59 | 1974 | Prospective | 35 | 40 % (14) | u | a, w, x, z |
| Dubois et al.18 | 1960 | Retrospective | 400 | 2.8% (11) | Not applicable | a, b, r |
- # initial treatment: high-dose prednisolone, including pulse therapy with methylprednisolone.
- * all patients under glucocorticoid therapy.
- Clinical factors: a. age, b. sex, c. race d. disease duration, e. Raynaud's phenomenon, f. oral ulcers, g. skin involvement h. lymphadenopathy, i. arthritis/ synovitis, j. serositis, k. lung involvement, l. renal involvement, m. neuropsychiatric SLE (NPSLE), n. hematologic involvement, o. vasculitis, p. antiphospholipid syndrome, q. antiphospholipid antibodies, r. seropositive for antibodies, s. SLE disease activity (SLEDAI), t. SLE damage score, u. high-dose prednisone or prednisolone, v. high initial prednisone or prednisolone dose, w. cumulative dose of prednisone or prednisolone, x. duration of glucocorticoid therapy, y. pulse therapy, z. use of immunosuppressant drugs, aa. hydroxychloroquine, bb. lipid-lowering agents, cc. Cushingoid body habitus variable, dd. septic arthritis, ee. Sjögren's syndrome, ff. hyperlipidemia, gg. hypertension, hh. osteoporosis, ii. Preeclampsia.
The effect of genetic ancestry and ethnicity on the incidence of ON in SLE patients is poorly characterized. Different cultures have varying genetic risks for developing SLE,38 and different ethnicities have varying rates of SLE patients developing ON. At present, specific genes or genetic markers specific to certain ethnicities modifying the risk of ON in SLE patients have not been clearly established.
Most of the studies estimating the prevalence of ON development in SLE patients were conducted in Asia (Table 1). The prevalence of ON in the general population is low. The average estimated number of annual prevalent cases of ON was 28.91 per 100,000 in Korea, based on the Medical Claims Database of the National Health Insurance Corporation.38 Although the prevalence of ON in the general population in the United States remains unclear, newly diagnosed patients are estimated to be between 20,000 and 30,000 every year in the United States. Cozen et al reported 7.69% Hispanic, 19.23% Black, and 57.69% white for the incidence of ON in American SLE patients.39 A prospective study at the University of Toronto Lupus Clinic has followed SLE patients since 1970. Among 235 ON patients, the distribution of ON was in 67.1% in Caucasian patients, 15.4 % in Black patients, 8.1% in Asian patients, and 9.4% in others ethnicities.40 Arranow et al showed that among eight African-American SLE patients, 75% of whom developed ON,41 suggesting that higher disease activity and higher doses of glucocorticoids in African-American SLE patients may be associated with the higher incidence of ON in patients of African-American origin. Demographic data of the prevalence of ON in SLE patients needs to be determined to estimate the burden of ON on different ethnicities.
Although ON is a prevalent complication in patients with rheumatic diseases as a whole, the incidence rate of ON is higher in patients with SLE compared to non-SLE patients (Table 2).17, 18, 23, 42 A retrospective study investigated that the frequency of ON as determined from discharge diagnoses of patients with various rheumatic diseases hospitalized at the Clinical Center of the National Institutes of Health from 1962 to 1977.43 A prospective MRI study showed that SLE was the most frequent underlying disease for ON patients who received glucocorticoid therapy from 1986 to 2009.44 In nationwide epidemiologic surveys conducted in Japan and Sweden in the 2000s,3, 45 ON occurred more frequently in patients with SLE than in any other rheumatic diseases requiring the administration of glucocorticoids. Therefore, glucocorticoids maybe not the only risk factor for the incidence of ON in SLE patients.
| Underlying diseases | Prevalence (%) |
|---|---|
| Systemic lupus erythematosus | 1.7-52 |
| Rheumatoid arthritis | 0.4-4.8 |
| Polymyositis/dermatomyositis | 0.1-4.9 |
| Granulomatosis with polyangiitis | 3.7 |
| Polymyalgia rheumatica | 3.3 |
| Mixed connective tissue disease | 2.6 |
| Polyarteritis nodosa | 2.1 |
| Giant cell arteritis | 1.2 |
| Sjögren's syndrome | 0.9-1.1 |
| Behçet's disease | 0.4 |
| Ankylosing spondylitis | 0.4 |
3.3 Pathogenesis
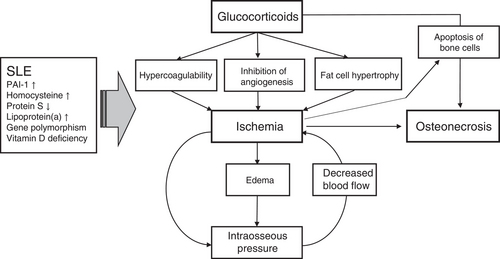
Osteonecrosis is a well-known comorbidity of patients with SLE.19-22, 42 The use of glucocorticoid therapy is a major risk factor for the incidence of ON in SLE patients.16, 17, 34, 35, 37, 40, 41, 43, 46-60 Multiple factors have been implicated in the pathogenesis of ON in patients who use glucocorticoids.9, 11-13 As glucocorticoids can induce changes in angiogenesis, intravascular coagulation, apoptosis of bone cells, and fat hypertrophy, glucocorticoid-mediated alterations may contribute to bone ischemia and necrosis by both intra- and extraluminal obliteration. Figure 1 summarizes the current understanding of the pathogenesis of ON in SLE patients, which is likely the combined result of multiple factors. However, the pathogenesis of ON in SLE patients remains unclear.
3.3.1 Hypercoagulability and inhibition of angiogenesis
Coagulation and congestion lead to decreased blood flow and oxygen delivery through the microvasculature, leading to an increased risk of developing ON. A genetic association between inherited thrombophilia, hypofibrinolysis, and ON has been established.61 It has been suggested that hypofibrinolysis results in increased clot formation, decreased blood flow, and a hypoxic environment within the bone structure, which may lead to ON. The dysregulation of coagulation and complement-related pathways has been found in patients with SLE and contributes to SLE disease activity.62 Furthermore, the presence of antiphospholipid antibodies in SLE can lead to a hypercoagulable state, which is believed to contribute to ON in SLE by promoting intravascular coagulation and congestion.63 However, the association between ON and antiphospholipid antibodies in SLE patients remains controversial. The role of antiphospholipid antibodies in the occurrence of ON has been found in only a few studies,23, 55, 64-66 while others have not exhibited any association with ON.67
Hypercoagulability can be compounded by the glucocorticoid-mediated inhibition of angiogenesis.68 Glucocorticoids can directly injure endothelial cells,69 enhance hypercoagulability,70 and inhibit angiogenesis by inducing apoptosis of bone marrow endothelial cells (BMECs).71 Glucocorticoids can inhibit angiogenesis by diminishing proliferating capillary haemangiomas,72 tube-like structure formation,73 new vessel formation,74 and angiogenic factor generation.75 This decrease in normal angiogenesis, caused by glucocorticoids, in the femoral head and bone tissue can lead to ON. Atsumi et al found that all patients with unilateral glucocorticoid-induced ON had abnormal superior retinacular arteries, small arterial penetration, and interruption of revascularization in the contralateral normal hips.76 Glucocorticoids, in combination with active inflammation or surgery, can also contribute to a hypercoagulable state.77 ON does not result from a single episode of impaired blood supply, but from a chronic blockade of microcirculation,78 the alteration of BMECs,79 and transcriptomic changes in bone microvascular endothelial cells.80, 81 Apoptotic endothelial cell death has been hypothesized to function as a mechanism for the capillary rarefaction in glucocorticoid-mediated hypertension.82 In turn, glucocorticoid-induced hypertension in the femoral head disturbs the blood flow in the femoral head vessels and aborts the repair process.12
3.3.2 Apoptosis of osteoblasts and osteocytes
The apoptosis of osteoblasts or osteocytes plays a crucial role in the pathogenesis of ON.83-85 Increased apoptotic osteoblasts or osteocytes have been observed in mice and humans under glucocorticoid therapy.86 It has been reported that apoptosis of osteocytes in the femoral head increases during the development of ON, and the percentage of apoptotic cells is significantly increased in the femoral heads of patients with glucocorticoid-induced ON. However, apoptotic bone cells are notably rare in bone from patients with alcohol-induced ON87, 88 and absent in those with trauma and sickle cell-induced ON. Apoptosis of the bone cells can result from increased inducible nitric oxide synthase (iNOS) and cytochrome C expression89 and aberrant metabolites in the synovial fluids.90 Although the death of bone cells is observed in the bone of patients with ON, the correlation between the apoptosis of bone cells and SLE-associated ON has not been clearly established.
3.3.3 Fat cell hypertrophy
Fat cell hypertrophy and fat emboli have been detected in rabbits after the exposure to high-dose cortisone for 5 months.91 Fat emboli can activate the complement pathway, deposit immune complexes, and increase intravascular coagulation,92 leading to ON. Glucocorticoids can skew the differentiation of bone marrow stem cells into adipocytes cells by upregulating adipocyte transcription factor gene expression and downregulating the gene expression of osteoblast transcription factors.91 Glucocorticoids increase lipid deposition leading to larger numbers of fat cells when compared to normal ratios of parenchyma in the mesenchymal stem cells of ON patients.93
In SLE patients, high-dose glucocorticoids result in an early and rapid drop in bone mass with a marked increase in body fat.94 These patients also have elevated levels of serum adiponectin95 and postmenopausal SLE women were found to have altered body composition and increased visceral adipose tissue.96 These factors, however, were not correlated with glucocorticoid dose. Intraosseous pressure is increased by the growth of fat cells in the intraosseous compartment and results in sinusoidal compression, leading to decreased perfusion of the bone, which may cause ON.97
4 RISK FACTORS FOR THE DEVELOPMENT OF ON IN PATIENTS WITH SLE
Glucocorticoid exposure is a well-recognized risk factor for ON in SLE patients (Table 1).15-17, 34, 35, 39-41, 43, 46-52, 54-59, 64-67, 98-113 In particular, the correlation between ON and the doses of glucocorticoid (GC) therapy has been identified in many studies (Table 1) and higher doses of GC therapy have been cited as one of the strongest predictive factors for developing ON. Twenty-four of 49 studies shown in Table 1 found a significant correlation between the dose of GC therapy and the occurrence of ON in patients with SLE. A meta-analysis suggests that high-dose glucocorticoid therapy may increase the risk of ON by as much as 10 times.22 Daily doses greater than 40 mg are associated with increased risk, with the incidence rate climbing by approximately 3.6% for every 10 mg increase.21 According to a 12-year longitudinal study, doses greater than 10 mg in combination with high-intensity GC use (≧80%) are associated with increased risk of osteonecrosis.113 A recent large cohort study that has followed SLE patients since 1970 utilized multivariate analysis to identify GC dose as a major predicative factor for the incidence of symptomatic ON.37 However, the association of the duration or route of GC therapy with the incidence of ON remains contentious. Intravenous pulses of methylprednisolone are often used to treat severe symptoms in SLE patients and have significantly higher immunosuppressive and anti-inflammatory effects compared to other delivery methods for glucocorticoids.114 Although high-dose intravenous pulses of methylprednisolone resulted in higher cumulative doses of GCs, its association with the occurrence of ON has conflicting evidence. An MRI study strongly linked GC pulse therapy with the early development of silent ON in SLE patients,51 and Massardo et al closely related GC pulse therapy to high-dose intravenous pulses of methylprednisolone.109 By contrast, several studies have also shown that GC pulse therapy is uncorrelated with ON.17, 23, 53, 54, 56, 63 A nationwide epidemiologic study in South Korea demonstrated that the route of glucocorticoid therapy does not affect SLE patient outcomes and oral and intravenous high-dose glucocorticoids carry equal risk.48 Consistently, in recent investigations, GC-related damages including ON were associated with high cumulative doses and high intensity of oral GCs and lower doses of GC use significantly diminished GC-induced damages even in the presence of methylprednisolone use.60, 113, 115 These studies also suggest that pulse methylprednisolone may not be an independent association factor for AVN.50, 54, 115 The duration of GC therapy was significantly longer in the AVN group compared to the control group.17, 50 However, Oinuma et al found that all osteonecrotic legions were detected very early, within 5 months after starting high-dose glucocorticoid therapy in 72 patients with SLE.52 This study suggests that silent ON can occur early after the onset of glucocorticoid therapy. During long-term, low-dose glucocorticoid therapy, there were no new cases of ON116 and spontaneous repairs were even observed.117 Although the etiology of ON in SLE patients is multifactorial, high-dose GC use remains a key risk factor for ON.
Studies show that SLE patients are at increased risk of developing ON as compared with patients who have other autoimmune conditions and receive similar doses of glucocorticoids. The risk factors for ON are not limited to glucocorticoids.19 Many studies have shown that ON in SLE patients is associated with various clinical factors, such as age, sex, disease duration, symptoms of SLE, laboratory factors, medications, and complications of SLE (Table 1 and Figure 2). Although the age at the time of glucocorticoid therapy is considered as a risk factor for ON,104 most adult studies identified that age is not a key risk factor for ON in SLE patients. The incidence of ON is relatively low in growing individuals but rapidly increases in adolescents and adults.44 There is a report showing a lower incidence of glucocorticoid-induced ON in pediatric SLE patients (<15 years old) than in adult SLE patients (>20 years old).104 Another dynamic MRI study identified higher blood supply in the growth plate of the femoral neck in pediatric SLE patients than adult SLE patients after glucocorticoid therapy.118
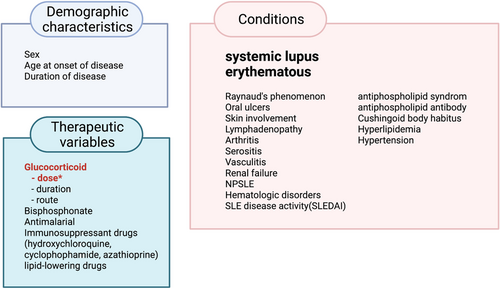
ONOsteonecrosis also has an increased incidence in SLE patients who have not been taking high-dose glucocorticoids.15 How SLE is an independent risk factor for ON has not yet been elucidated. Disease-related factors, including Raynaud's phenomenon and vasculitis, also determine ON risk among patients with SLE.51, 65, 100, 102, 106, 110 A long-term follow-up study found that SLE recurrence is a risk factor for ON. Among 106 SLE patients, SLE recurrence occurred in 131 joints. The mean time from SLE recurrence to the appearance of new osteonecrotic lesions was 6.2 months. In one single-center retrospective study with 88 consecutive SLE patients, a high antiphospholipid score was a risk factor for idiopathic ON in SLE patients.119 There are conflicting results for the association of antiphospholipid antibodies with ON (Table 1). Some studies showed a positive correlation,20, 66, 101 while many other studies showed no significant correlation with the incidence of ON in SLE patients.39, 46, 49, 55, 58, 99, 100, 102, 103, 106 A higher frequency of Cushingoid body habitus showed an association with the incidence of ON in SLE patients.40, 46, 49, 55, 109
It is well known that glucocorticoids induce iatrogenic metabolic syndrome. Due to this, hyperlipidemia is considered to be an important risk factor for glucocorticoid-induced ON in SLE. Twelve prospective studies of glucocorticoid-induced ON in SLE patients have been conducted (Table 1). In addition to a high dosage of glucocorticoids, hyperlipidemia was commonly seen as a risk factor for ON in prospective studies. One prospective study of 78 SLE patients treated with high-dose prednisolone, showed a correlation between elevated levels of triglyceride and an increased incidence of ON.100 In addition, the chronic systemic inflammation or high levels of oxidized LDLs in SLE patients can affect the skeletal phenotype by indirectly influencing bone cells.120 ON can be caused by the partial or total disruption of blood flow to the femoral head, and SLE patients tend to develop ON through a similar mechanism. SLE patients with ON are more likely to have hypofibrinolytic 4G polymorphism of the plasminogen activator inhibitor-1 gene, methylenetetrahydrofolate reductase gene mutation with a higher concentration of homocysteine, low protein S values, and higher lipoprotein(a) levels than controls.50 SLE patients are invariably encouraged to avoid sunshine exposure, as this increases the risk of vitamin D deficiency. One juvenile SLE study reported that vitamin D deficiency was significantly associated with subjects who had or developed ON.23 However, the contribution of the autoimmune pathophysiology in SLE disease to the development of ON has not yet been fully clarified.
Disease-modifying anti-rheumatic drugs (DMARDs), such as hydroxychloroquine, azathioprine, and sulfasalazine, are becoming more prevalent in the management of autoimmune conditions. However, there are a few studies investigating the impact of DMARDs on rates of developing ON in SLE patients. Six of the eight studies shown in Table 1 reported an inverse correlation between the use of hydroxycholoroquine and ON development, although this difference was not statistically significant. A recent retrospective study of the Taiwanese population involved 1472 children newly diagnosed with SLE, and 1364 of these patients had DMARDs as a part of their management.98 Although the patients did not have statistically different usages of DMARD's between the ON and non-ON groups, the cumulative duration of hydrocychloroquine use was significantly correlated with ON. Association between the use of biologic DMARDs, including Bellimumab121 and Rituximab, and ON in SLE patients has not been established yet, although Rituximab, an anti-CD20 antibody, has been suggested as a cause of medication-related ON of the jaw.122 The use of DMARDs may result in the use of lower doses of glucocorticoids in SLE patients, which may affect the incidence of ON in SLE patients. Overall, the current research is insufficient to understand the impact of DMARD's on ON in SLE patients and further research needs to be conducted to fully understand the correlation between DMARDs and ON.
Recent studies have also revealed some new genetic risk factors for ON in SLE patients. Several association studies using targeted next-generation sequencing technologies identified single nucleotide variations (SNVs) for developing ON in SLE patients (Table 3). Kim et al. have identified that Asp258Asp (exon 6: rs1549758) and Glu298Asp (exon 7: G895T: rs1799983) polymorphisms in the nitric oxide synthase 3 (NOS3) gene may be related to ON susceptibility in SLE patients under the recessive model.123 A case-control study demonstrated that rs3813946 in the 5′-UTR, rs311306 in intron 1, and the rs17615 in exon 10 of the CR2 (complement receptor type 2, complement C3d Receptor 2) gene.124 A recent study has also identified that SNPs for ON risk in SLE patients include NOS3 (exon 6: c.814G > A, p.E272K), Collagen Type II Alpha 1 Chain (COL2A1, c.3508G > A, rs41263847: exon 29: c.1913C > T: p.T638I, exon 28: c.1706C > T: p.T569I, and rs371445823: exon 8: c.580G > A: p.A194T, exon 7: c.373G > A: p.A125T),125 and CR2 (rs45573035: exon 2: c.200C > G: p.T67S).125 Most of the association studies were conducted in Asia and used a small sample size. Since ethnic difference has been identified across many association studies of genetic polymorphisms in ON, further studies for evaluating these SNPs in other ethnicities are needed. More elaborately designed and larger-scale studies may clarify more SNPs and their functions in the ON susceptibility of SLE patients. The contribution of genetic predisposition to SLE etiology has been increasingly appreciated, and over 100 susceptibility loci for SLE risk have been identified.126, 127 However, the genetic predispositions associated with ON susceptibility in SLE patients, including ancestry effects, have not yet been demonstrated. Polymorphism of T786C NOS3 in the promoter was associated with idiopathic ON128 but not with ON in SLE patients,123 suggesting the influence of different ethnic groups on genetic variation. Investigation of the prevalence of genetic risk factors associated with ON susceptibility in both the general population and in patients of varying ancestries, is a consideration for a future study. To summarize, understanding the impact of genetic variation may provide new insight into ON and ultimately lead to new treatment methods.
| Gene name | Genotype, rs# | Location | References |
|---|---|---|---|
| NOS3 (nitric oxide synthase 3) | rs1549758 | exon 6 | 123 |
| G895T: rs1799983 | exon 7 | 123 | |
| c.814G > A: p.E272K, | exon6 | 125 | |
| COL2A1 (Collagen type II alpha-1 gene) | c.1913C > T: rs41263847: p.T638I | exon 29 | 125 |
| c.1706C > T: p.T569I | exon 28 | 125 | |
| c.580G > A: rs371445823: p.A194T | exon 8 | 125 | |
| c.373G > A: p.A125T | exon 7 | 125 | |
| CR2 (Complement receptor type 2) | rs3813946 | 5′-UTR | 124 |
| rs311306 | intron 1 | 124 | |
| G639A: rs17615 | exon 10 | 124 | |
| c.200C > G: rs45573035: p.T67S | exon 2 | 125 |
5 GLUCOCORTICOID-INDUCED OSTEONECROSIS ANIMAL MODELS
Animal models of ON of the femoral head (ONFH) are indispensable to the understanding of the mechanism, treatment, and prevention modalities for ON of the femoral head. Different animal models for glucocorticoid-induced ON have been generated. Of all experimental models, rabbits are most commonly used to establish glucocorticoid-induced ON.129 However, these studies were not able to develop joint collapse, which was mainly explained by the lower mechanical loading onto the weight-bearing joints. Administration of a single glucocorticoid proved to be an efficient way to induce ON in rabbits or mice,130, 131 resulting in the incidence of ON with this methodology ranging from 10% to 43%. It is necessary to develop an SLE mouse model with a high incidence of glucocorticoid-induced ON to elucidate the prevention and treatment efficiency of the pharmacological therapy strategies.
5.1 Clinical course and diagnosis
5.1.1 Pain
The earliest clinical symptom of ON is bone pain that limits motion, is persistent, and is aggravated by weight-bearing and activity.132 When patients have this persistent bone pain, providers typically order imaging studies to further understand the etiology of this bone pain.
5.1.2 Radiology
Conventional radiography is the first-line investigation for the diagnosis of ON.13 ON is usually diagnosed by X-ray and magnetic resonance imaging (MRI); however, imaging studies at present do not have the sensitivity to screen for ON at earlier and more manageable stages. Scanning with MRI is the most sensitive modality for diagnosing ON, although it is costly.133 The Canadian and American guidelines for SLE recommend radiography as the initial imaging modality for patients suspected of ON, and magnetic resonance imaging (MRI) or single-photon emission computed tomography (SPECT) if X-rays are not informative134,.135 Whole-body short-TI inversion recovery MRI (STIR MRI) has shown promising results in early studies, suggesting that it may become one of the most sensitive and rapid tools for detecting ON lesions at early stages. One study used whole-body STIR MRI to evaluate ON in 40 adolescents with SLE who received glucocorticoid treatment; there they found seven patients (17.5%) with ON in the knee, hip, and ankle and 37 ON lesions overall.136 In addition, MRI can quantify the area of ON.137 A 20-year retrospective study showed that among 30 SLE patients, more than half of those treated with glucocorticoids were already in late-stage ON when clinical manifestations arose.138 However, at present, there are no universal screening guidelines for ON to catch disease at an early stage when conservative and minimally invasive treatments are indicated. The routine MRI screening of SLE patients may facilitate the early detection of ON; however, other constraints, such as financial considerations and resource scarcity, need to be evaluated.
5.2 Staging
Classically, ON presents in the femoral head, but it can lead to isolated lesions in other locations such as the jaw or knees. It can also be multifocal. Detection and classification, using one of the four classification systems (Ficat, UPenn, ARCO, and Japanese Orthopedic Association), are crucial to choosing the appropriate treatment modality. However, there are still no guidelines on the imaging screening of ON in SLE patients.
5.2.1 Classification
The classification system of ONFH is crucial to deciding the appropriate clinical intervention. Four classification systems are used to classify ONFH, regardless of etiology: the Ficat Classification (used most commonly), the University of Pennsylvania System, the Association Research Circulation Osseous (ARCO) System, and the Japanese Orthopedic Association system (Tables 4-8).139 Notably, the Association Research Circulation Osseous (ARCO) classification system has undergone revisions recently to eliminate stage 0 and divide stage III into stages IIIA (femoral head depression less than or equal to 2 mm) and stage IIIB (femoral head depression more than 2 mm; see Table 8).140 Future directions of classification may include combining findings from digital subtraction angiography (DSA) and MRI to establish the staging of intraosseous circulation obstruction based on the blood supply status of the femoral head.141 This staging is based on changes in blood circulation, which can better provide guidance for the treatment strategy to preserve the femoral head preservation, especially in young patients.141
| Stage | Radiographic Findings |
|---|---|
| 1 | None (only evident on magnetic resonance images) |
| 2 | Diffuse sclerosis, cysts (visualized on radiographs) |
| 3 | Subchondral fracture (crescent sign; with or without head collapse) |
| 4 | Femoral head collapse, acetabular involvement, and joint destruction (osteoarthritis) |
| Stage | Criteria |
|---|---|
| 0 | Normal radiograph, bone scan, and magnetic resonance images |
| I |
Normal radiograph. Abnormal bone scan and/or magnetic resonance images A: Mild (< 15% of femoral head affected) B: Moderate (15% to 30% of femoral head affected) C: Severe (> 30% of femoral head affected) |
| II |
Cystic and sclerotic changes in femoral head A: Mild (< 15% of femoral head affected) B: Moderate (15% to 30% of femoral head affected) C: Severe (> 30% of femoral head affected) |
| III |
Subchondral collapse without flattening (crescent sign) A: Mild (< 15% of articular surface) B: Moderate (15% to 30% of articular surface) C: Severe (> 30% of articular surface) |
| IV |
Flattening of femoral head A: Mild (< 15% of surface and < 2 mm of depression) B: Moderate (15% to 30% of surface and 2 to 4 mm of depression) C: Severe (> 30% of surface and > 4 mm of depression) |
| V |
Joint narrowing or acetabular changes A: Mild B: Moderate C: Severe |
| VI | Advanced degenerative changes |
| ARCO Stage | Image Findings | Description |
|---|---|---|
| I | X-ray normal, MRI abnormal | A band lesion of low signal intensity around the necrotic area is seen on MRI. A cold spot is seen on bone scan. No changes are seen on plain radiographs. |
| II | X-ray abnormal, MRI abnormal | Osteosclerosis, focal osteoporosis, or cystic changes are seen in the femoral head on plain radiographs or CT scan. Still there is no evidence of subchondral fracture, fracture in the necrotic portion, or flattening of the femoral head. |
| III | Subchondral fracture on X-ray or CT | Subchondral fracture, fracture in the necrotic portion, and/or flattening of the femoral head is seen on plain radiography or CT scan. |
| IIIA (early) | Femoral head depression ≤2 mm | |
| IIIB (late) | Femoral head depression > 2 mm | |
| IV | X-ray osteoarthritis | Osteoarthritis of the hip joint with joint space narrowing, acetabular changes, and destruction are seen on plain radiographs |
| Stage | Finding |
|---|---|
| 1 |
Demarcation line, subdivided by relationship to weight-bearing area (from medial to lateral) 1A 1B 1C |
| 2 | Early flattening WITHOUT demarcation line around necrotic area |
| 3 |
Cystic lesions, subdivided by site in the femoral head 3A (medial) 3B (lateral) |
| Radiologic Findings | ARCO Stage in 1994 | ARCO Stage in 2019 |
|---|---|---|
| Preclinical and preradiographic | 0 | |
| Evident change on MRI | I | I |
| Evident change on X-ray | II | II |
| Subchondral fracture | III | |
| Head collapse 2 mm | IIIA | |
| Head collapse > 2 mm | IIIB | |
| Joint space narrowing or acetabular changes | IV | IV |
5.2.2 Musculoskeletal manifestations of SLE
Glucocorticoid-induced ON frequently develops at the femoral head in the hip9 but many other sites, such as the knee, shoulder, ankle, and hand can also be affected simultaneously.142-144 Multifocal ON, a rare variant appearing in only 3.3% of all patients with ON, is defined as the presence of osteonecrotic lesions in three or more separate anatomical sites.144 Intriguingly, SLE patients receiving long-term glucocorticoid treatment have a higher incidence of multifocal ON.37, 145, 146 A multicenter study of patients with multifocal ON reported that 38% (38 of 101) of the multifocal ON patients had a previous SLE diagnosis.146 Of these patients, chronic exposure to glucocorticoid therapy was the most common risk factor (91%). All 101 patients with multifocal disease had femoral head involvement. The most common additional sites were in the knee (96%), shoulder (80%), and ankle (44%) with seven other joints also being implicated. The clinical manifestations associated with multifocal ON appeared to be similar to those with non-multifocal involvement. Patients with SLE who develop multifocal ON tend to be younger, have several SLE clinical manifestations and serological abnormalities, and most have been exposed to glucocorticoid and immunosuppressive agents.147, 148
5.3 Treatment
The treatment of glucocorticoid-induced ON includes non-operative management and surgical approaches (Figure 3). Treatments range from ON prophylactic medications such as bisphosphonates, statins, and anticoagulants, which show mixed efficacy, to surgical interventions for more advanced diseases such as core decompression with and without bone grafting, rotational osteotomy, and hip replacement. The choice of treatments is dependent on the stage of the ON, the size of the lesion, the age of the patient, and the patient's co-morbidity.
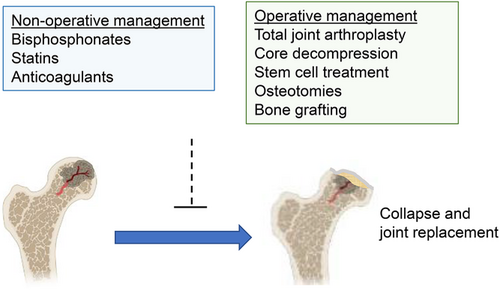
5.3.1 Non-operative management
There is no uniformly accepted treatment for ON.149 Various pharmacological agents, such as bisphosphonates, statins, and anticoagulants, have been used to promote viable bone growth within necrotic lesions and alleviate pain and the progression of ON. The effects of these drugs are limited, and additional research is needed to establish the efficacy of these individual agents before routine use of any of them is recommended. However, reports analyzing the effect of non-operative management for glucocorticoid-induced ON in SLE patients are limited. Further studies are needed in order to clarify the effects of these treatments on the incidence of ON in SLE patients,
Bisphosphonates
Open-label studies show that bisphosphonates have shown efficacy in preventing disease progression in ON and in delaying the progression to surgery,149, 150 although the efficacy of alendronate, a bisphosphonate, in ON is still controversial. A study by Agarwala et al of 60 ON patients, 10 of whom had SLE, showed that alendronate reduces pain, improves function, and delays ON progression.151 One study analyzed the time from a diagnosis of ON to hip replacement as a marker for the ability of alendronate to minimize disease progression. In the placebo control group, seven hips had a total hip arthroplasty (THA) in the first 12 months and 12 hips by 18 months. In the alendronate group, only six patients progressed to THA and the proportion of hips that developed collapse was also significantly smaller. In contrast, another two-year, multicenter, prospective, randomized, double-blind, placebo-controlled trial study stated that there was no statistical difference in progression to THA between placebo (13%) and bisphosphonate (15%) usage.152 Overall, the evidence of the effects of bisphosphonates on glucocorticoid-induced ON with SLE is mixed, and further studies are needed to evaluate the effect of various bisphosphonates on glucocorticoid-induced ON for SLE patients.
Statins
Hyperlipidemia has also been associated with ON.8 Lipid-lowering agents such as atorvastatin and Lipitor have been tested as treatments for ON in a randomized controlled clinical trial153 and in animal models.154 Lipid-lowering agents are thought to prevent ON by reducing the differentiation of marrow pluripotent cells into fat cells, which may lead to increased intraosseous pressure. One database review of 284 patients found a reduced incidence of glucocorticoid-induced ON among individuals who were on a statin before the initiation of glucocorticoid treatment.155 Only 1% of patients develop ON over the 5-year period. Prospective clinical studies are needed to clarify the effects of statins on glucocorticoid-induced ON with SLE.
Anticoagulants
Anticoagulants, such as warfarin or enoxaparin, have not significantly prevented the progression of disease in ON patients with SLE despite a decreasing tendency to progress to THA.156 One study evaluated 60 patients with SLE receiving high-dose glucocorticoid treatment, treating about half of the patients for anticoagulation with warfarin.157 Although the differences were not statistically significant, fewer patients in the warfarin group developed ON compared with the control group (21% versus 33%). Similarly, a prospective study of 35 hips with Ficat stage 1 and two cases of ON with thrombophilia reported that with enoxaparin (60 mg/day for three months) only 20% of patients showed progression of the disease over a 2-year period.158 Another prospective study using warfarin in SLE showed no statistically significant effect of anticoagulant therapy on the prevention of glucocorticoid-induced ON.159 To summarize, the data suggest that anticoagulants did not significantly alter the progression nor affect the prevention of ON.
5.3.2 Surgical Management
Total hip arthroplasty has become most the effective treatment for ON, while non-arthroplasty treatment options for the management of ON have produced variable results in SLE-associated ON.160 As ON typically affects younger adults, as compared to osteoarthritis, the preservation of the hip joint and the early treatment are equally important for preventing the progression to collapse. Therefore, the interventions including core decompression, stem cell therapy, osteotomies, and non-vascularized or vascularized bone grafting have been selected in the early stages of ON, with total joint replacement being reserved for end stage disease.
Total joint arthroplasty
Total hip arthroplasty is often seen as the last resort and the most aggressive treatment of ON, typically reserved for late-stage ON when cortical collapse seems imminent. Despite the advanced stage of the disease, the outcomes of this procedure have been fairly positive. Among SLE patients, the annual numbers of total hip joints arthroplasties, partial hip joints arthroplasties, and total knee arthroplasties have shown a statistically significant increase over time.161 Figgie et al reviewed the population-based rate of joint replacement in SLE patients from 1991 to 2005 and demonstrated that the rate of arthroplasty in patients with SLE has been increased from 17% in 1991 to 38% in 2005.162 However, the rate of THA for ON has been decreased from 53% in 1991 to 24% in 2005.162 Chen et al used The PearlDiver patient records database and reported a 190% of increase in THA for ON in patients with SLE from 2007 to 2015.163 THA plays a particularly important role in the treatment of ON in patients with SLE as these patients progress to late stages much more rapidly than other etiologies of ON. Musculoskeletal pain and function are of great concern for these patients.
Core decompression
Core decompression (CD), the most frequently used procedure, preserves the structure of the hip and relieves bone pain.164 It can reduce intraosseous pressure, penetrate area hardened by fibrotic changes, promote the growth of blood vessels along the tunnel into the femoral head, enhance the formation of new bone, and delay ON.165 CD is suitable for early- stage ON patients.166, 167 In addition, CD can provide better clinical or imaging results.164 However, there is an element of selection bias in these results, given that the majority of patients receive CD at early stages of the disease.164 An additional long-term study suggested that CD can be an effective alternative treatment for an early stage of glucocorticoid-associated ON in SLE patients, especially relieving pain and postponing progression to THA.168 During the past decade, the efficacy of CD has been improved and techniques such as single large-diameter drilling and multiple small-diameter drilling with and without bone grafting have been developed.169 It has been suggested that CD plus autologous bone therapy or cytotherapy is a better way to reduce the failure rate of conservative treatment in patients with early and mid-stage ON.170, 171
Stem cell treatment
The application of stem cell treatment, which many previously considered experimental,172 has gained accumulating evidence for clinical improvement.173-175 A 30-year follow-up prospective randomized study based on 125 consecutive patients, bone marrow cell transplantation can be an effective treatment for early-stage femoral head ON. Bone marrow cell transplantation can delay the progression of the disease, reduce the incidence of collapse, and avoid joint replacement.176 The study also found that after excluding some factors that may affect clinical and radiological results, CD and bone marrow mesenchymal stem cells (BM-MSCs) implantation was an effective method to reduce the THA conversion rate of ON patients, especially for the early-stage patients. However, CD and BM-MSCs did not prevent the progress of ARCO staging.176 A recent systematic review and meta-analysis also found that compared with CD treatment alone, the use of MSCs in early stages of ON patients lowered the rates of disease progression and failure and led to fewer minor complications.177, 178 Implantation of ex-vivo expanded BM-MSCs, in combination with CD, has shown promise as a treatment for ON.179 In a study conducted by Mardones et al, five ON patients received ex-vivo expanded MSCs and the hip function of all patients significantly improved. Concentrated autologous bone marrow aspirate transplantation (CABMAT) slows the progression of ON to THA with only minor side effects.180
The beneficial effect of stem-cell therapy has also been shown in the SLE patient population as well. Yoshikoga et al demonstrated that CABMAT significantly improved pain and Harris Hip Scores in eight of nine hips from 18 ON patients with SLE.181 Mid-term follow up for CABMAT showed that ON patients with SLE who received CABMAT had lower conversion rates to THA.182 A recent case report using autologous bone marrow aspirate concentrate (BMAC) injections to treat an 18-year-old female SLE patient with glucocorticoid-related ON in bilateral knees showed that after 24 months follow-up, the patient had improved in function and had pain relief.183 Bone marrow aspirate transplantation has the advantages of minimal invasiveness, low cost, simplicity, and the ability to be used in combination to augment other treatment methods as well.173
Osteotomies
Transtrochanteric rotational osteotomy, an osteotomy procedure predominantly performed in Asia, shifts the weight-bearing area to a field of healthy bone, relieving the pressure of weight on necrotic bone.184 Studies show that the 5-year and 10-year hip survival rate of ON patients after transtrochanteric rotational osteotomy is satisfactory in both Asian patients and non-Asian populations.184 After proper selection of patients, accurate surgical procedures, and appropriate postoperative rehabilitation treatment, transtrochanteric rotational osteotomy can be used as an effective hip protection measure for young patients, people with active symptomatic ON, and ON patients with SLE185,.186
Vascularized and non-vascularized bone grafting
Bone-grafting, most commonly as an autogenous vascularized bone or vascularized bone harvested from the fibula or iliac crest, has also been described in the literature as a treatment method for ON.187, 188 Both vascularized and non-vascularized bone grafts have been shown to improve outcomes for ON patients by improving joint function and delaying joint repair surgery.189, 190 A recent systematic review of 15 studies demonstrated that compared with core decompression and non-vascularized fibular bone grafting, free vascularized fibular transplantation is a better treatment option, especially in young patients who have early-stage ON, before collapse.189 Eighty hips belonging to 50 SLE patients who underwent free vascularized fibular grafting for ONFH were followed for more than 2 years (average 4.3 years) and the hip score improved in all patients.190 Numerous studies have shown that a non-vascularized fibular allograft combined with CD and bone grafting is a cost-effective way of improving the survival rate for an early stage of ONFH, delaying disease progression, and improving the quality of patients' lives.191, 192
Complication of surgical intervention
Although total joint arthroplasty for SLE patients can generally obtain good or excellent clinical results and improve the quality of life, SLE patients receiving total joint arthroplasty have a higher complication rate than non-SLE counterparts, which requires various measures to prevent.193-195 Both SLE and long-term glucocorticoid use increase the risk of perioperative complications such as wound infection.160 Lin et al showed a dose-dependent relationship between preoperative GC treatment and postoperative complications and mortality in SLE patients.196 In patients with SLE, disease activity and infection are the two main causes of death postoperatively.197 However, the recent studies showed a lower infection rate, which is likely due to careful patient selection and increased provider precautions.160, 163, 193 Although the use of GCs in SLE patients may increase the risk of wound infection, proper care can improve the infection rate. Another consideration is thrombophilia or hypercoagulability, which are often prevalent in SLE patients. In addition, the prevalence of antiphospholipid syndrome is increased from 1% to 5% in healthy individuals to approximately one third of SLE patients.198 The adequate use of prophylactic anticoagulation following orthopedic surgery may diminish the incidence of thromboembolic complications.199 It is also crucial to consider that ON patients typically have these components implanted at a younger age, meaning that they must retain these implants for much longer than their non-autoimmune counterparts. The risks of implant failure and other complications compound as a result.200 The complication rate reported in the literature varies greatly, making it difficult to ascertain the true risk of post-THA surgical complications in the SLE population.
5.4 Potential candidates for the management of ON
5.4.1 Hyperbaric oxygen therapy
Hyperbaric oxygen (HBO) therapy increases the level of tissue oxygenation,201 which can, in turn, lead to promoting fibroblast proliferation, collagen synthesis, and angiogenesis.202 Several studies have suggested that HBO therapy improves the symptom of ON by potentially lowering intraosseous pressure within the femoral head and by improving microcirculation.203 Bosco et al showed that HBO therapy can diminish inflammatory cytokines and ROS in patients with ON.204 HBO therapy has been suggested to be an effective option for patients with early-stage ON.205 A recent meta-analysis for 10 studies using HBO therapy as the treatment for ON has identified a significant clinical benefit of HBO therapy on ON in Asian populations.206 It is necessary to clarify the effect of hyperbaric oxygen therapy on glucocorticoid-induced ON in the future.
5.4.2 Natural compounds
The potential efficacy of several natural compounds on ON has been suggested. These natural compounds have a modulatory effect on bone cells – promoting bone formation and inhibiting bone resorption. Genistein aglycone, an isoflavone widely found in soybeans and seen as a natural alternative to selective estrogen receptor modulators,207 protected mice from both ovariectomy-induced bone loss and glucocorticoid-induced bone loss208-210 and showed a protective effect on bone loss in postmenopausal women.211, 212 Bitto et al also found that, in some cases, genistein aglycone also showed a positive outcome for methylprednisolone-induced necrotic deterioration of the femoral head.209 Vinpocetine, a natural compound extracted from the leaves of Phyllostachys pubescens,213 has also shown a protective role in ON of the femoral head in rat models.214 However, the protective effect of natural compounds on ON has not been tested in clinical human studies and needs to be further studied in ON patients.
6 CONCLUSION
Osteonecrosis is a complication that can often cause joint pain and loss of function within the joints leading to physical disability for many SLE patients. SLE patients with ON do not typically respond to conservative treatment and eventually require joint replacement. However, the pathogenesis of ON in SLE patients is still controversial. It is important to note that ON develops only in a subset of SLE patients who received high-dose glucocorticoids. This discrepancy suggests that there are underlying patient-specific factors that govern susceptibility to ON in the setting of high-dose GCs. It remains unclear what patient-specific factor is associated with the incidence of ON in SLE patients. As the underlying reason for susceptibility of ON in SLE patients remains unclear, it is imperative to identify the risk factors that precipitate ON in SLE patients. As shown in Table 1, a high glucocorticoid dosage is a strong association factor for the incidence of ON in SLE patients. Thus, many studies suggested that lowering the dose of oral glucocorticoids can minimize the incidence of ON in SLE patients. However, Chen et al demonstrated that SLE patients who received low-dose glucocorticoid therapy showed a higher risk of GC-related damages than SLE patients who did not receive GC therapy.113 Therefore, identifying a safe dose for preventing GC-related damage would be critical. As there is no cure for SLE patients and glucocorticoid therapy is important for the management of SLE, identifying the lowest effective doses of glucocorticoids in combination with other agents will be required to minimize the incidence of ON.
Multifocal ON is often found in SLE patients with ON and is characterized by the involvement of multiple separate anatomic sites. It has been suggested that there is a strong relationship between multifocal disease and glucocorticoid therapy. However, it is difficult to interpret the potential effect of glucocorticoid use because of the many variables, including dosage, duration of treatment, and route of administration. Between 80% and 90% of patients tested with multifocal ON had hypofibrinolysis or thrombophilia or both. Because of the high incidence of coagulation disorders in patients with ON, it is difficult to evaluate the difference between patients with multifocal ON and those with less musculoskeletal involvement. It is necessary to conduct epidemiological studies to clarify the etiologic factors of multifocal ON.
High-dose glucocorticoids are a key risk factor for ON in SLE patients. The death of bone cells is evident in ON bone and the biologic responses to glucocorticoids in bone cells have been extensively studied. The dose and duration of glucocorticoids are significantly associated with the likelihood of developing ON. However, the responses to glucocorticoids in ON patients can be altered by the differences between individuals in glucocorticoid sensitivity, which are influenced by multiple factors including genetic predisposition, metabolic factors, and other factors affecting blood supply.14 Therefore, systematic evaluation of the risk factors in ON patients is warranted for prevention and interventions.
The majority of ON patients are in the middle-aged population who are physically active. Thus, it is critical to improve pain and the function of affected joints and to delay total joint replacement surgery. Recent advances in stem-cell therapy allow its usage in many orthopedic procedures.215 MSC-based therapy demonstrates promising benefits in animal models, but its therapeutic application is currently limited due to a lack of understanding of the tissue regenerative function of human MSCs in vivo. Autologous MSCs from healthy donors appear to exhibit immunomodulatory and tissue-protective effects after transplantation.216 In contrast, autologous MSCs from SLE patients do not carry immunosuppressive properties.217, 218 In addition, osteogenesis from the bone marrow aspirate of SLE patients is significantly impaired compared to healthy donors.219 Thus, it is crucial to develop a systematic approach to determine whether or not the BM-MSCs of SLE patients with ON still have normal osteogenic potential.220
While arthroplasty is indicated for advanced-stage ON, the described non-surgical and surgical interventions have been shown to be effective in the early stages of ON. It has also been suggested that early detection of ONFH in the pre-collapse stage is associated with an improved and more favorable clinical outcome. Thus, awareness of the need for prevention of glucocorticoid-induced ON in the early stage is increasing and advanced techniques and interventions to detect ON early in SLE patients under glucocorticoid therapy are required.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health 5R01 AR069562; 5R01 AR073156 (to P.M.K.H.), Japan Research Foundation for Clinical Pharmacology (K.K), and by Giammaria and Sabrina Gluliani Foundation. Figures were generated using Biorender.
AUTHOR CONTRIBUTIONS
K.K., H.C., and K.-H.P.-M. were associated with study conceptualization; K.K., H.C., M.K., and I.S. wrote and prepared the original draft of the manuscript. E.S. and K.-H.P.-M. reviewed and edited the final manuscript. K.-H.P.-M. acquired funding. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




