Bridging viral hepatitis and liver cancer: Emerging concepts in pathogenesis and therapeutic innovation
Joint authors. Keyin Zheng, Aimin Jiang and Zhengrui Li have contributed equally to this work and share first authorship.
Abstract
Background
Viral hepatitis, particularly hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, represent the predominant etiological factors for hepatocellular carcinoma (HCC) worldwide. HBV and HCV drive hepatocellular malignant transformation through complex molecular mechanisms that are both distinct and overlapping. Comprehensive elucidation of these mechanisms, particularly the role of viral-mediated remodeling of the tumor microenvironment, is crucial for developing novel preventive and diagnostic strategies as well as personalized therapeutic approaches.
Aim
This review aims to systematically elucidate the key molecular mechanisms underlying HBV- and HCV-related HCC development and progression (including virus-specific pathways and common pathways), to explore the translational potential of these mechanisms in clinical medicine, and to provide perspectives on future research frontiers.
Results
This review systematically elucidates the pathogenic mechanisms of HBV- and HCV-related HCC and provides comprehensive analysis of the common molecular mechanisms underlying viral hepatitis-to-HCC transformation. For HBV-related HCC, we focus on analyzing the following oncogenic mechanisms: genomic instability caused by HBV DNA integration, oncogenic effects of HBV proteins, and the impact of virus infection-mediated tumor microenvironment remodeling on immune responses. For HCV-related HCC, we focus on exploring the following oncogenic mechanisms: oncogenic mechanisms of viral proteins, virus infection-mediated metabolic disorders, functional dysregulation of immune cells in the microenvironment, and virus-induced hepatic fibrosis. Furthermore, we thoroughly investigated the common mechanisms underlying viral hepatitis-to-HCC transformation, including the construction of pro-inflammatory factor networks in chronic inflammatory microenvironments, virus-induced epigenetic alterations, and genomic instability. Based on current research, we further discuss future research directions and perspectives in this field.
Conclusion
This review systematically elucidates the pathogenic mechanisms of HBV- and HCV-related HCC and provides comprehensive analysis of the common molecular mechanisms underlying viral hepatitis-to-HCC transformation, with particular emphasis on the remodeling effects of viral infection on the HCC microenvironment, which hold significant clinical implications for developing novel preventive strategies, diagnostic biomarkers, and personalized therapeutic approaches. Through systematic analysis of the long-term effects of virus infection-induced epigenetic reprogramming in HCC development and progression, combined with multi-omics data to construct HCC risk prediction models, our findings provide scientific evidence for the development of early screening and precision treatment strategies. Meanwhile, investigating the relationship between viral integration patterns and HCC prognosis, and developing novel molecular classification methods, will facilitate the design of more individualized and precise treatment regimens for patients. Additionally, utilizing cutting-edge artificial intelligence technologies and developing innovative research approaches such as viral hepatitis-related liver organoid models will also provide novel insights and methodologies for reducing the incidence and mortality of viral hepatitis-related HCC.
1 INTRODUCTION
Viral hepatitis, a major global public health concern, has been established as one of the primary aetiologies of hepatocellular carcinoma (HCC).1 Among viral hepatitis-related HCC cases, hepatitis B virus (HBV) and hepatitis C virus (HCV) are identified as the most significant pathogenic agents. HBV and HCV infections are widely acknowledged as definitive risk factors for the development and progression of HCC.2 Among all liver cancer cases, HCC accounts for approximately 90%. HBV-related HCC accounts for approximately 50% of all liver cancer cases, representing about 60% of HCC cases in Asia and Africa, while comprising approximately 20% of HCC cases in Western countries. In North America, Japan and Europe, chronic HCV infection represents the most common underlying liver disease among HCC patients.3 The lifetime risk of developing HCC among chronic HBV carriers ranges from 10% to 25%.1 Among patients with HCV-related cirrhosis, the annual incidence of HCC ranges from 0.5% to 10%.1 The disease progression of chronic HBV infection leading to HCC is typically characterised by four stages: immune-tolerant phase, immune-active phase, inactive phase and reactivation phase. Studies have demonstrated that approximately 20%–30% of patients in the inactive phase progress to the reactivation phase, characterised by high-level HBV replication, which subsequently promotes the progression from chronic hepatitis to liver fibrosis, cirrhosis and ultimately HCC.4 Chronic hepatitis C is diagnosed when HCV RNA remains detectable for 6 months after initial infection. This persistent infection can lead to progressive hepatocyte fibrosis, with approximately 20%–30% of chronic hepatitis C patients progressing to cirrhosis within a 20-year disease course.5
HBV and HCV promote the occurrence and progression of HCC through multiple molecular mechanisms, involving complex interactions between viral and host factors. HBV-mediated hepatocarcinogenesis primarily occurs through the following mechanisms: (1) integration of viral DNA into the host genome, (2) oncogenic effects of viral proteins and (3) dysregulation of host immune responses. HCV-induced hepatocarcinogenesis involves the following mechanisms: (1) oncogenic effects of viral proteins, (2) virus-mediated metabolic disorders, (3) aberrant immune responses and (4) induction of liver fibrosis. Persistent chronic inflammation induces continuous secretion of pro-inflammatory factors by cells, which act on hepatocytes and stromal cells, thereby mediating immune evasion, inhibiting apoptosis, promoting tumour cell proliferation and inducing epithelial–mesenchymal transition (EMT) and angiogenesis.6-8 Oxidative stress leads to excessive accumulation of reactive oxygen species (ROS), which promotes the occurrence and development of HCC through pathways such as enhancing telomerase activity, inducing DNA mutations, exacerbating genomic instability and causing mitochondrial dysfunction.9, 10
Despite significant progress in the study of hepatitis virus-related HCC, numerous critical scientific questions still remain to be elucidated. HBV and HCV exhibit markedly different carcinogenic mechanisms: HBV primarily exerts its carcinogenic effect by integrating viral DNA into the host genome, whereas HCV mainly promotes carcinogenesis by inducing metabolic disorders in host cells. Nevertheless, these two viruses also exhibit several common characteristics in their carcinogenic processes: (1) both can suppress host immune function; (2) both encode viral proteins with carcinogenic activity; (3) both can promote HCC development through inducing epigenetic modifications; and (4) both can disrupt host genome stability. It remains to be determined whether targeting these common carcinogenic mechanisms could provide better prevention strategies for virus-related HCC and improved treatment options for patients co-infected with both hepatitis viruses. Furthermore, while we have gained some understanding of the carcinogenic mechanisms of HBV and HCV, the complex interactions among carcinogenic processes within the tumour microenvironment—such as the positive feedback loops between inflammatory mediators, oxidative stress and metabolic disorders that promote carcinogenesis; the bidirectional regulation between epigenetic alterations and viral proteins; and the intricate regulatory networks formed by multiple signalling pathways—remain substantial gaps in our comprehension of HCC pathogenesis. Additionally, host factors play an equally crucial role in the pathogenesis of HCC, further complicating our understanding of the disease.
For instance, individuals with characteristics such as male sex, advanced age, concomitant metabolic diseases and a family history of HCC have a significantly increased risk of developing HCC. The relative contributions of these host factors and viral factors to HCC development, as well as the potentially complex interactions between them, remain inadequately studied. Based on preliminary explorations of the carcinogenic mechanisms of HBV and HCV, researchers have identified potential early diagnostic markers, such as oncoproteins, inflammatory mediators, ROS concentrations, liver fibrosis indicators and DNA methylation status. Simultaneously, several potential therapeutic targets have been identified, such as those targeting viral proteins, inflammatory regulators, oxidative stress products, epigenetic alterations, immune cell functions or immune checkpoints, metabolic regulatory enzymes and core signalling pathways. However, it should be noted that whether these therapeutic targets and diagnostic markers can truly be applied clinically requires further research and validation. Further research is necessary to develop more precise early diagnostic markers and more effective therapeutic targets, ultimately providing patients with more accurate and personalised treatment strategies.
This review comprehensively examines the molecular mechanisms underlying the progression from viral hepatitis to HCC, with a focus on the following aspects: (1) Mechanisms of HBV-related HCC development, encompassing genomic instability caused by HBV DNA integration, the carcinogenic effects of HBV proteins (particularly HBx and HBsAg) and the impact of virus-mediated tumour microenvironment remodelling on immune responses; (2) Mechanisms of HCV-related HCC development, emphasising the carcinogenic mechanisms of viral proteins, metabolic disorders, dysfunction of immune cells in the microenvironment and virus-induced liver fibrosis; (3) Common molecular mechanisms involved in the transformation from viral hepatitis to HCC, including the establishment of pro-inflammatory factor networks in the chronic inflammatory microenvironment, microenvironment-mediated epigenetic alterations and genomic instability; (4) Future research directions, encompassing virus–host interactions, biomarker development, prevention and treatment strategies, molecular and cellular heterogeneity studies and the development of novel experimental models, with a particular focus on deciphering the spatiotemporal heterogeneity of the tumour microenvironment to develop novel therapeutic strategies. In-depth elucidation of these molecular mechanisms, particularly how viral infections reshape the HCC microenvironment, holds significant clinical implications for developing novel prevention strategies, diagnostic markers and personalised treatment regimens, potentially yielding new insights and approaches to reduce the incidence and mortality of viral hepatitis-related HCC.
2 MECHANISMS OF HBV-RELATED HCC DEVELOPMENT
Current research suggests that the key carcinogenic mechanisms of HBV-related HCC primarily include (Figure 1): (1) host genomic instability caused by HBV DNA integration; (2) carcinogenic effects mediated by HBV X protein (HBx) and hepatitis B surface antigen (HBsAg); and (3) tumour microenvironment remodelling induced by viral infection and its subsequent effects on immune responses.
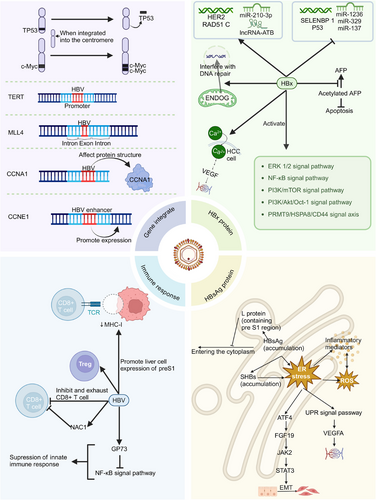
2.1 HBV DNA integration
2.1.1 HBV DNA integration leads to host genomic instability and mutations
HBV DNA integration can predispose host chromosomes to translocations during non-homologous recombination, potentially leading to structural variations in the human genome and consequently increasing genomic instability.11 The majority of studies have demonstrated that, compared to non-tumour tissues, HBV integration sites in tumour tissues exhibit a higher concentration and show a tendency to cluster at specific gene loci.12 Furthermore, HBV integration sites in tumour samples are more frequently located in intergenic regions, potentially affecting promoter areas and subsequently promoting aberrant gene expression. In contrast, HBV integration sites in normal samples are predominantly found in intronic regions.13 Elevated expression of TERT and MLL4 genes is strongly associated with tumour development and progression. HBV genome integration commonly occurs in the telomerase reverse transcriptase (TERT) promoter region, resulting in upregulation of TERT expression.14 Similarly, HBV integration is prevalent in the exonic regions of the MLL4 gene, contributing to elevated MLL4 mRNA expression levels.15
2.1.2 HBV DNA integrates into the vicinity of oncogenes or genes that promote the occurrence and development of cancer, thereby activating the expression of such genes
HBV DNA integration into regions adjacent to oncogenes has the potential to activate oncogene expression. For instance, when integration occurs in proximity to the proto-oncogene c-Myc, it may lead to its overexpression, potentially promoting the development of HCC.16 HBV integration can potentially promote cancer initiation and progression by altering the expression of genes adjacent to its integration sites. For example, mutations in CCNA2 and CCNE1 are capable of inducing uncontrolled cell proliferation, replication stress and chromosomal rearrangements. HBV integration may contribute to carcinogenesis by directly altering the structure of CCNA2-encoded proteins or by facilitating CCNE1 overexpression through HBV enhancer sequences.17
2.1.3 HBV DNA integration can induce chromosomal rearrangements and activate distant oncogenes
In a subset of HCCs, HBV integration has been observed to induce chromosomal rearrangements, particularly when the integration site is located in centromeric or telomeric regions, which may lead to the activation of distant oncogenes. For example, when integration occurs at the centromere, it can potentially cause large deletions in chromosome 17p, including the loss of the tumour suppressor gene TP53, or result in extensive amplification of genes in the 8q region of the chromosome, such as the amplification of the proto-oncogene MYC.17
2.2 Carcinogenic effects of HBV proteins
2.2.1 HBx
Activation of multiple signalling pathways
HBx is a multifunctional regulatory protein encoded by the HBV genome, which plays a crucial role in the life cycle and pathogenesis of HBV. HBx protein contributes to the occurrence and progression of HCC by activating multiple signalling pathways, including PI3K/AKT and NF-κB: (1) HBx enhances the expression of p90 ribosomal S6 kinase 2 (RSK2) by activating the extracellular signal-regulated kinase (ERK) 1/2 signalling pathway. As an important cancer-associated protein, the upregulation of RSK2 significantly facilitates the initiation and progression of HCC.18 (2) HBx facilitates the occurrence and progression of HCC by inhibiting ferroptosis through the regulation of the HBx/PRMT9/HSPA8/CD44 signalling axis. Specifically, HBx upregulates PRMT9 expression, which subsequently promotes the overexpression of HSPA8 and CD44. Studies have demonstrated that downregulation of CD44 can significantly enhance ferroptosis; consequently, HBx inhibits ferroptosis by activating this signalling axis, ultimately facilitating the occurrence of HCC.19 (3) HBx activates the NF-κB signalling pathway through interaction with NF-κB, thereby enhancing the expression of its downstream target genes, including IFN-γ, c-Myc, TBK1 and IL-6. The aberrant expression of these genes collectively contributes to the initiation and progression of HCC.20 (4) HBx upregulates the expression of GRP78, which in turn activates Smad4 and upregulates MAN1B1, ultimately leading to the activation of the PI3K/mTOR signalling pathway. This cascade significantly facilitates the initiation and progression of HCC.21 (5) The ectopic expression of HBx activates the PI3K/Akt/Oct-1 signalling pathway, resulting in the dissociation of C/EBPβ from the RHAMM gene promoter, thus enhancing RHAMM expression. The upregulation of RHAMM expression significantly facilitates the metastasis and colonisation of HCC cells.22
Regulation of host gene expression
HBx, a multifunctional protein, can influence the expression of host genes through various mechanisms, thereby promoting the initiation and progression of HCC. HBx can promote the expression of oncogenes such as HER2 and inhibit the expression of tumour suppressor genes like p53,23, 24 while also broadly regulating the expression of various genes related to HCC initiation and progression in the nucleus. For instance, HBx promotes the expression of RAD51 C through nuclear interaction, while RAD51 C, in turn, enhances HBV replication, forming a positive feedback loop.25 Furthermore, HBx inhibits the promoter activity of selenium-binding protein 1 (SELENBP1), resulting in decreased SELENBP1 expression. Studies have demonstrated that SELENBP1 expression is significantly lower in HCC tissues compared to normal liver tissues, suggesting that SELENBP1 suppression may be a crucial mechanism through which HBV promotes HCC development.26
Regulating host epigenetic modifications
Regarding the regulation of host epigenetic modifications, HBx exhibits significant regulatory effects, particularly in promoting the initiation and progression of HCC by modulating the expression patterns of host microRNAs (miRNAs). Research has demonstrated that in HBV-induced HCC, miR-210-3p is the sole significantly upregulated miRNA. Further experimental evidence has confirmed that miR-210-3p can inhibit the expression of PTPN 18 while concurrently upregulating the expression level of HBx in HBV-infected Huh 7/NTCP cells.27, 28 Furthermore, HBx can indirectly induce the upregulation of AFP expression by suppressing the expression of miR-1236 and miR-329.29 Moreover, HBx can also suppress the expression of miR-137, which exerts tumour-suppressing effects, thereby facilitating the initiation and progression of HCC.30 Notably, HBx can also enhance the expression of transforming growth factor β-activated long non-coding RNA (lncRNA-ATB). As an oncogenic lncRNA, the elevated expression of lncRNA-ATB can significantly promote HCC metastasis and invasion.31 HBx protein can further facilitate HCC progression by modulating the methylation modification levels of host RNA. The expression of phosphatase and tensin homolog (PTEN), a key tumour suppressor gene, is tightly regulated by HBV. Research has demonstrated that HBV can significantly elevate the N6-methyladenosine (m6A) modification level of PTEN mRNA, resulting in decreased PTEN protein expression, consequently activating the PI3K/AKT signalling pathway and promoting the initiation and progression of HCC.32
Induction of oxidative stress and mitochondrial dysfunction
HBx demonstrates the capacity to inhibit cellular autophagy, resulting in sustained damage to hepatocyte nuclear DNA (nDNA) mediated by mitochondrial endonuclease G (ENDOG), consequently significantly increasing the instability of the host genome. Under normal physiological conditions, cellular autophagy effectively prevents ENDOG from traversing the mitochondrial membrane and entering the nucleus. However, when HBx inhibits autophagy, ENDOG can cross the mitochondrial membrane and enter the nucleus, thereby interfering with the repair process of nDNA and ultimately leading to increased instability of the host genome.33 HBx disrupts the oxidative phosphorylation process in mitochondria, consequently inhibiting ATP generation and severely impacting the normal energy metabolism of hepatocytes. HBx induces excessive generation of ROS and lipid peroxidation through blocking proton transfer. Excessive accumulation of ROS not only induces gene mutations but also significantly enhances the carcinogenesis process in hepatocytes. Furthermore, elevated ROS levels promote the release of inflammatory factors, including IL-1β, IL-6 and TNF-α, thus activating STAT3 and MAPK signalling pathways. Concurrently, ROS stimulates NF-κB to induce the production of additional inflammatory cytokines. These abundantly generated inflammatory mediators, in turn, further enhance ROS release, establishing a vicious positive feedback loop, which ultimately results in persistent hepatocyte damage and carcinogenesis.6
Promotion of cell proliferation and inhibition of apoptosis
Upregulation of STC2 can inhibit cell apoptosis, whereas HMGA2 promotes the expression of STC2. Although ectopic expression of HBx induces DNA damage and oxidative stress, it concurrently upregulates HMGA2 expression, thus preventing ROS-induced apoptosis in HBV-infected cells.34 This perpetuates cells in a chronic state of stress, which may constitute one of the critical mechanisms underlying HCC development. The binding of phosphatase PTEN to pro-apoptotic protein caspase-3 is essential for maintaining normal cell apoptosis, and disruption of this binding can facilitate cancer development. Studies have demonstrated that acetylated AFP can mediate this blocking process, and HBx enhances HCC progression by inhibiting deacetylation, consequently increasing the content of acetylated AFP.35 This finding elucidates new potential targets for the treatment of HBV-related HCC.
Promotion of EMT
HBx augments pro-inflammatory cytokine-mediated EMT by facilitating the release of ROS.6 Furthermore, HBx can induce STAT3-mediated upregulation of EMT-related gene expression in HCC cells, consequently accelerating HCC invasion and metastasis.36 These mechanisms collectively constitute a crucial molecular basis for HBV-mediated HCC progression.
Upregulation of VEGFA promotes angiogenesis
HBx is capable of elevating intracellular calcium concentration, thereby upregulating the expression of high-mobility group box 1 (HMGB1) in HCC cells through calcium-mediated signalling cascades. Subsequently, HMGB1 promotes the expression of vascular endothelial growth factor (VEGF), ultimately leading to enhanced tumour angiogenesis.36, 37 This finding elucidates a potential new intervention strategy for inhibiting HBV-related HCC angiogenesis.
2.2.2 HBsAg
Induction of endoplasmic reticulum stress
HBsAg is composed of three envelope proteins: small (S), middle (M) and large (L). Overexpression of L protein, particularly L protein containing the pre-S1 region, inhibits HBsAg secretion, resulting in its accumulation in the endoplasmic reticulum (ER). This accumulation elicits ER stress, initiating a series of biochemical reactions, including elevated ROS release and production of inflammatory mediators. Prolonged ER stress disrupts normal liver physiological functions, enhances cellular instability and may ultimately contribute to HCC development.38 This mechanism elucidates the crucial link between chronic HBV infection and HCC occurrence.
Promotion of EMT
Accumulation of small surface antigens (small hepatitis B surface protein [SHBs]) in the ER elicits ER stress, which subsequently activates the transcription factor ATF4. ATF4 facilitates the upregulation and secretion of fibroblast growth factor 19 (FGF19). Secreted FGF19 triggers EMT in HCC cells through the activation of the JAK2/STAT3 signalling pathway.39 This finding unveils a significant link between HBV surface antigens and HCC invasiveness, offering new insights for developing targeted therapeutic strategies.
Promotion of angiogenesis
ER stress induced by SHBs can activate the unfolded protein response (UPR) signalling pathway, thus promoting the expression and secretion of vascular endothelial growth factor A (VEGFA).40 This mechanism elucidates a direct link between HBV infection and HCC angiogenesis, offering new potential targets for inhibiting HBV-related HCC angiogenesis.
2.3 Host immune response
2.3.1 Suppression of innate immune response
The persistent inflammatory microenvironment and progressive liver damage induced by HBV infection are key pathogenic mechanisms underlying the development of HCC. Moreover, chronic HBV infection significantly increases the risk of HCC due to the presence of a long-term inflammatory microenvironment. The suppression of the host's innate immune response enables HBV to acquire enhanced immune evasion capabilities, facilitating the transition from acute to chronic infection, and ultimately promoting the occurrence and progression of HCC. Studies have demonstrated that HBV infection can upregulate the expression level of Golgi protein 73 (GP73), consequently weakening the host's innate immune response by inhibiting the nuclear factor κB (NF-κB) signalling pathway.41
2.3.2 T cell exhaustion
Chronic HBV infection can induce persistent liver tissue damage and repair, resulting in progressive liver fibrosis and substantially increasing the risk of cirrhosis. Clinical data suggest that approximately 90% of HCC patients have a history of liver cirrhosis. The immunosuppressive state mediated by HBV promotes persistent viral infection, wherein T cell exhaustion serves as a crucial immunological mechanism contributing to the progression of chronic infection to HCC. During the dynamic progression of HBV infection to HCC, the proportion of effector CD8+ T cells demonstrates a pattern of initial increase followed by decrease, whereas the proportion of regulatory T cells (Tregs) exhibits a trend of initial decrease followed by increase. This dynamic change in immune cell subsets indicates that HBV may suppress the host's antiviral immune response by inducing effector T cell exhaustion and promoting Treg cell expansion.42 Recent studies have revealed that HBV can further enhance its suppression of host immune responses by upregulating the expression of nucleus accumbens-associated protein 1 (NAC1), which inhibits CD8+ T cell activation and proliferation.43 This mechanism offers a novel molecular basis for understanding persistent HBV infection.
2.3.3 Evading immune surveillance by downregulating cell surface MHC-I molecule expression
Under normal circumstances, a healthy and functional immune system can recognise and eliminate virus-infected abnormal cells and tumour cells through the MHC-I pathway, preventing their progression to HCC. Studies have shown that HBV can promote the expression of the large protein preS1 region in hepatocytes, thereby suppressing the expression of MHC-I on the cell surface. For HBV-infected abnormal hepatocytes, the failure to eliminate these cells leads to persistent viral infection, with acute infection gradually evolving into chronic infection, and ultimately progressing to malignant transformation. For newly formed tumour cells infected with HBV and undergoing malignant transformation, the downregulation of surface MHC-I expression facilitates their evasion of immune surveillance by cytotoxic T lymphocytes, leading to uncontrolled proliferation of tumour cells and progression into HCC.44
3 MECHANISMS OF HCV-RELATED HCC DEVELOPMENT
Research has confirmed that the carcinogenic mechanisms of HCV-related HCC primarily involve the following aspects (Figure 2): (1) HCV-encoded viral proteins (including core protein, NS5A protein and NS3 protein) exhibit significant carcinogenic potential; (2) HCV-mediated dysregulation of lipid and glucose metabolism; (3) HCV-induced immune response dysregulation; and (4) HCV-mediated liver fibrosis progression.
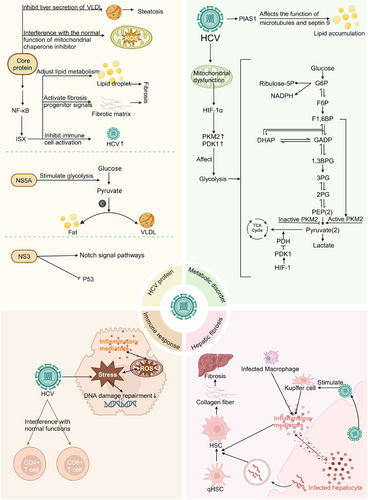
3.1 Carcinogenic effects of HCV proteins
3.1.1 Core protein: Inducing oxidative stress and promoting lipid metabolism disorders
The HCV core protein induces lipid metabolism disorders by upregulating the expression of intestine-specific homeobox (ISX). ISX, functioning as a proto-oncogene protein, forms an HCV core–ISX axis with the HCV core protein, leading to upregulation of the kynurenine signalling pathway and subsequent disruption of normal lipogenesis. Moreover, this axis transcriptionally activates multiple genes involved in lipid and glucose metabolism, ultimately resulting in metabolic disorders in HCV-infected cells.45 The HCV core protein also impairs the mitochondrial electron transport system by disrupting the normal function of mitochondrial chaperonin, thereby inducing oxidative stress and ultimately promoting HCC development.46
3.1.2 NS5A: Reprogramming cellular metabolism
The HCV non-structural protein NS5A reprograms host cell metabolism, consequently promoting HCV replication. NS5A modulates glucokinase activity, leading to dysregulated glycolysis and consequently increasing carbon utilisation for lipogenesis and the secretion of very low-density lipoproteins (VLDL). This altered metabolic pattern not only facilitates HCV infection of hepatocytes but also creates favourable conditions for HCV-mediated HCC development.47
3.1.3 NS3: Upregulation of oncogenes and downregulation of tumour suppressor genes
The HCV NS3 protein possesses the capability to enhance the activity of the Notch signalling pathway. Given the critical role of the Notch pathway in maintaining cellular homeostasis and regulating cell development, the NS3 protein may promote tumour initiation and progression through the abnormal activation of the Notch signalling pathway.48 Furthermore, studies have shown that the NS3 protein can interact with the tumour suppressor p53 and inhibit its expression, potentially serving as another mechanism promoting tumourigenesis.49
3.2 HCV-induced metabolic disorders
3.2.1 Promotion of lipid accumulation leading to steatosis
HCV promotes lipid accumulation by upregulating the expression of the STAT1 protein inhibitor (PIAS1), which subsequently affects microtubules and Septin 9, thereby creating a favourable environment for viral replication.50 Additionally, the HCV core protein suppresses the secretion of VLDL from the liver, leading to decreased VLDL levels. Furthermore, studies have shown that hypobetalipoproteinemia is associated with steatosis and a higher incidence of cancer.51 Moreover, steatosis may be associated with the progression of liver fibrosis, which in turn could potentially promote the development of cirrhosis and HCC.52
3.2.2 Glucose metabolism dysregulation
HCV infection can result in mitochondrial dysfunction, subsequently leading to the activation of hypoxia-inducible factor 1α (HIF-1α). This process not only upregulates the expression of HIF-1-mediated glycogenesis-related genes but also remodels the glycolytic pathway, ultimately enhancing the metabolic plasticity of tumour cells.10 HIF-1 reprograms cellular glycolysis and biosynthetic networks by upregulating the expression of its downstream target genes, notably pyruvate kinase M2 (PKM2) and pyruvate dehydrogenase kinase 1 (PDK1), thereby providing sufficient energy supply and biosynthetic precursors for sustained cancer cell proliferation. PKM2 exhibits structure-dependent dual functions: its active tetrameric conformation promotes ATP generation, while the inactive dimeric conformation enhances the pentose phosphate pathway, resulting in the production of ribose-5-phosphate and NADPH, which serve as crucial precursors for DNA replication and lipid synthesis. PDK1 induces the Warburg effect, a hallmark metabolic characteristic of cancer cells, by inhibiting pyruvate entry into the tricarboxylic acid cycle and promoting its conversion to lactate, thus optimising the metabolic network to meet the robust energy and biosynthetic demands of tumour cells.53
3.3 HCV-induced immune response
3.3.1 Chronic inflammation and oxidative stress
HCV-related HCC typically develops in a highly oxidative microenvironment,54 which strongly suggests a close association between chronic HCV infection and oxidative stress. Studies have demonstrated that the HCV core protein modulates the release of mitochondrial ROS by enhancing calcium ion uptake. These ROS, in turn, stimulate the production of inflammatory mediators, establishing a positive feedback loop that continuously amplifies the inflammatory response.55 Another study suggests that HCV-induced oxidative stress possesses carcinogenic potential, causing DNA damage and impairing DNA repair systems, which results in the accumulation of DNA damage, as well as persistent inflammatory responses and liver injury.10
3.3.2 T cell function inhibition
CD8+ T cells play a pivotal role in anti-tumour immune responses by directly recognising and lysing tumour cells, as well as secreting various effector cytokines that synergistically regulate other immune cells.56-58 HCV infection impairs the anti-tumour effector function of CD8+ T cells through abnormally activating the tumour suppressor protein p53, interfering with early metabolic reprogramming and signal transduction pathways, and inducing mitochondrial dysfunction and glucose metabolism disorders.59 Helper T lymphocytes (CD4+ T cells) can indirectly inhibit tumour proliferation by secreting anti-tumour cytokines and synergistically activating other immune cells, as well as maintain anti-tumour immune responses through MHC-I-independent pathways when tumour cells downregulate major histocompatibility complex-I (MHC-I) to evade CD8+ T cell immune surveillance. Studies have demonstrated that HCV impairs CD4+ T cell function through multiple molecular mechanisms, including elevating intracellular ROS levels, interfering with intracellular signal transduction, inhibiting DNA damage repair, disrupting mitochondrial DNA (mtDNA) and nDNA integrity, promoting accelerated telomere shortening and disrupting mitochondrial protein homeostasis.55
3.4 Mechanisms of HCV-induced liver fibrosis
Cirrhosis represents a significant risk factor for HCC, and advanced liver fibrosis exhibits a strong positive correlation with the risk of HCC development. HCV facilitates the activation of hepatic stellate cells (HSCs) from their quiescent state via multiple mechanisms and enhances their secretion of collagen fibres. These collagen fibres accumulate in the extracellular matrix, subsequently leading to the development of liver fibrosis: (1) HCV infection induces hepatocytes to secrete various mediators, including soluble factors such as cytokines and chemokines, as well as miRNA-enriched exosomes. These mediators act on quiescent HSCs (qHSCs), facilitating their transformation into an activated state. (2) HCV viral particles released from infected hepatocytes directly interact with liver-specific macrophages—Kupffer cells—stimulating the release of pro-inflammatory mediators and chemokines, thus activating qHSCs. (3) Moreover, HCV-infected liver macrophages secrete substantial amounts of pro-inflammatory factors and chemokines, further enhancing the activation of qHSCs.7
4 COMMON MECHANISMS FROM VIRAL HEPATITIS TO HCC
The common molecular mechanisms underlying the progression from viral hepatitis to HCC primarily encompass the following (Figure 3): (1) Chronic inflammatory microenvironment: This is characterised by persistent cycles of hepatocyte injury and regeneration, secretion of pro-inflammatory mediators and production of ROS; (2) Epigenetic alterations: These include changes in DNA methylation patterns, aberrant histone modifications and dysregulation of non-coding RNA expression, which subsequently affect gene expression profiles and promote tumour initiation and progression; (3) Genomic instability: The accumulation of DNA damage and telomere dysfunction leads to significantly increased genomic instability. These factors interact through intricate signalling networks, synergistically driving the malignant transformation from viral hepatitis to HCC.
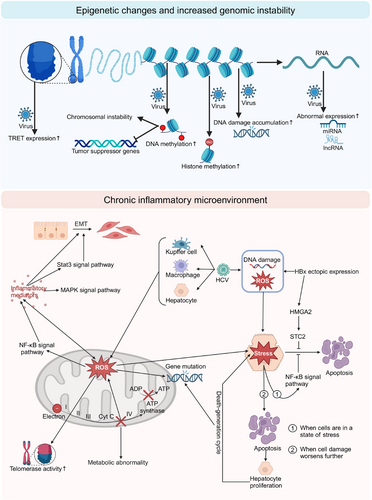
4.1 Chronic inflammatory microenvironment
4.1.1 Persistent hepatocyte injury and regeneration
Upon viral infection of hepatocytes, the body initiates an inflammatory response to protect cells from damage. However, as HBV and HCV infections frequently progress to chronic hepatitis, the persistent inflammatory response can exert long-term deleterious effects on the liver. The cellular inflammatory response can be categorised into four distinct stages: basal state, stress state, apoptotic state and necrotic state. Cells typically endeavour to impede progression to more severe states. When cells sustain damage and enter a stress state, the NF-κB signalling pathway is upregulated, thereby inhibiting cellular apoptosis. Consequently, cells persist in a prolonged stress state, continuously exposed to an environment of oxidative stress. This persistent oxidative stress promotes the development of liver fibrosis and cirrhosis. Furthermore, ROS can contribute to the development of HCC by enhancing telomerase activity, inducing DNA mutations and promoting genomic instability.9 However, if cellular damage further intensifies, it may culminate in cell apoptosis or necrosis. To maintain liver function, the body stimulates hepatocyte proliferation to replenish damaged cells.60 This perpetual cycle of cell death and regeneration may augment the accumulation of genetic mutations, thereby further promoting the development of HCC.
4.1.2 Secretion of pro-inflammatory factors
The HBx protein can induce oxidative stress, mitochondrial dysfunction and activate multiple signalling pathways, while the HBsAg protein can induce ER stress. HBV promotes the secretion of pro-inflammatory factors through these complex mechanisms. Similarly, HCV can also promote the release of inflammatory mediators through interactions with Kupffer cells, hepatocytes and macrophages in the liver. These pro-inflammatory mediators (including IL-6, IL-1β, IL-10, TNF-α, TGF-β and IL-18) can promote the development and progression of HCC through various mechanisms, primarily including: mediating immune evasion by acting on hepatocytes and stromal cells, inhibiting apoptosis, promoting tumour cell proliferation and inducing EMT and angiogenesis.6-8
4.1.3 ROS generation
As previously mentioned, both the HBx protein of HBV and the core protein of HCV can lead to significant ROS production in infected hepatocytes. HCC associated with these two viral infections typically develops in a highly oxidative microenvironment,54 strongly suggesting a close relationship between chronic hepatitis virus infection and oxidative stress. ROS activate various signalling pathways (such as NF-κB and STAT3) to induce the production of numerous inflammatory mediators (such as IL-1β, IL-6 and TNF-α) in the body. These inflammatory mediators, in turn, further promote ROS production, creating a positive feedback loop that continuously exacerbates hepatocyte damage.6 ROS can promote tumour growth through various mechanisms: (1) inducing DNA damage, leading to mutation accumulation; (2) inducing mitochondrial dysfunction, causing abnormalities in energy metabolism, glucose metabolism and lipid metabolism,10 which is particularly important for HCV replication; (3) activating the NF-κB pathway and inhibiting the production of apoptotic factors. Through these complex interactions, ROS significantly promote tumour initiation and progression.
4.2 Epigenetic alterations
4.2.1 DNA methylation abnormalities
Extensive research has demonstrated that aberrant DNA methylation plays a pivotal role in the initiation and progression of cancer.61 Studies have revealed that DNA methylation levels demonstrate a progressive increase as HCC advances.62 DNA methylation can lead to increased chromosomal instability and facilitate tumourigenesis through the silencing of tumour suppressor genes.63 Notably, hypermethylation of the E-cadherin gene promoter results in suppressed gene expression, a phenomenon closely associated with HCC progression. Both HBV and HCV can independently induce DNA methylation and, furthermore, synergistically enhance the methylation process. This synergistic effect of HBV and HCV promotes p53-dependent DNA methylation, resulting in the upregulation of mesenchymal markers and concomitant downregulation of epithelial markers, potentially facilitating the EMT process.64
4.2.2 Histone modifications
Studies on viral hepatitis-related HCC have demonstrated a significant upregulation in the expression of genes that regulate histone kinase activity.65 In HBV-related HCC, the level of histone H3 lysine 4 trimethylation (H3K4me3) is significantly elevated, and this enhancement in epigenetic modification is closely associated with tumour development and progression.66 Research suggests that HCV can downregulate the expression of the homeobox (HOX) gene family by decreasing the level of histone H2A monoubiquitination. Abnormalities in HOX gene expression products are strongly associated with tumourigenesis.67
4.2.3 Aberrant expression of non-coding RNAs
Comparative transcriptomic analysis of HCC tissues and normal liver tissues has revealed widespread aberrant expression of lncRNAs and miRNAs in HCV-infection-related or HBV-infection-related HCC tissues.68-70 HBV and HCV can modulate the host gene expression profile by regulating non-coding RNA expression, a mechanism that may play a pivotal role in the transformation process from viral hepatitis to HCC.71
4.3 Genomic instability
4.3.1 Accumulation of DNA damage
Chronic HCV and HBV infections can stimulate excessive production of ROS in host cells, which not only directly induces nDNA damage but also indirectly results in host DNA damage by disrupting mtDNA. Moreover, these viruses can interfere with DNA repair mechanisms, leading to the accumulation of DNA damage and ultimately aggravating genomic instability.10 Furthermore, the HBx protein encoded by HBV can enhance the accumulation of DNA damage by degrading the Smc5/6 complex, which suppresses viral replication in human hepatocytes.72
4.3.2 Telomere dysfunction
Integration of the HBV genome frequently occurs in the promoter region of the TERT gene, significantly enhancing TERT expression.73 HCV infection primarily stimulates hepatocytes to produce large amounts of ROS, leading to oxidative damage to telomere structure and function, thereby affecting telomere stability and integrity. Abnormal telomere elongation enables tumour cells to evade replicative senescence, acquiring the ability for unlimited proliferation, which is one of the key features in HCC development and progression.
5 FUTURE RESEARCH DIRECTIONS AND PROSPECTS
Based on current research progress, future research directions and prospects in this field can focus on the following aspects (Figure 4): (1) Systematically elucidate virus–host interaction networks and their regulatory mechanisms in HCC development using single-cell omics technologies and virus–host protein interaction network analysis platforms; (2) Develop molecular marker systems for early diagnosis and prognosis prediction, including HCC risk prediction and prognostic assessment models integrating multi-omics data3; Develop targeted prevention and therapeutic intervention strategies, with a focus on exploring gene editing therapeutic approaches for viral integration hotspots and their clinical translation4; Conduct in-depth analysis of molecular and cellular heterogeneity characteristics of viral hepatitis-related HCC and their clinical significance5; Establish and optimise novel experimental models and advanced research technology platforms, with emphasis on the development and application of humanised mouse models and liver organoid systems.
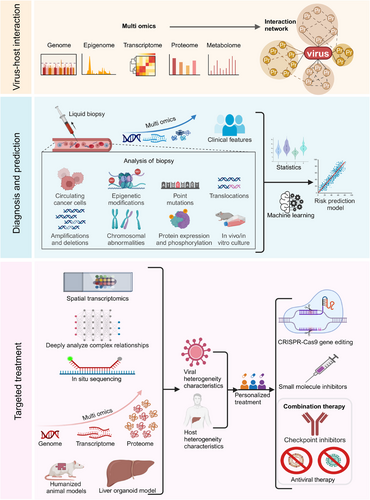
5.1 In-depth study of virus–host interactions and their critical role in HCC development
Future research should further explore the complex network of interactions between viral and host factors, including virus–host protein interactions, virus-induced alterations in host gene expression and modulation of immune responses. This foundational knowledge can serve as a basis for developing novel prevention and treatment strategies, potentially impeding the progression from viral hepatitis to HCC.
5.1.1 Utilising single-cell omics technologies to study the impact of viral infection on hepatocyte heterogeneity and tumour microenvironment
Single-cell omics technologies, including single-cell sequencing, single-cell proteomics and single-cell metabolomics (SCM), provide powerful tools for the in-depth analysis of hepatocyte subpopulation heterogeneity and their dynamic evolution following viral infection. Compared to traditional high-throughput omics analyses that examine common characteristics of mixed cell populations, single-cell multi-omics analysis can reveal the multi-layered heterogeneity of HBV/HCV-infected hepatocytes at the genomic, transcriptomic, proteomic and metabolomic levels, thereby enabling precise identification of distinct hepatocyte subpopulations and their functional states, tracking of cell fate decisions following viral infection, and the potential discovery of novel tumour subtypes and therapeutic drug targets.74-79 Among these approaches, single-cell RNA sequencing (scRNA-seq) technology not only characterises different cell types but also identifies which cell subpopulations express specific genes and quantifies their expression levels; however, this technology exhibits low detection rates for low-abundance viral RNA, and future approaches may consider integration with other technologies, such as clustered regularly interspaced short palindromic repeats (CRISPR), which could potentially achieve selective enrichment of HBV transcripts.80, 81 Single-cell proteomics can directly detect protein abundance and post-translational modification states, thereby circumventing the issue of insufficient correlation between mRNA and protein levels. Next-generation sequencing (NGS)-based genomic sequencing technology can measure multiple modalities within the same cell, achieving high throughput through amplification and barcoding; however, it relies on customised targeted affinity reagents and can detect only a limited variety of proteins. In contrast, single-cell mass spectrometry (scMS) methods can detect thousands of proteins and post-translational modifications without requiring targeted affinity reagents, enabling the discovery of novel biological properties; however, they have high technical requirements (requiring protein preservation throughout the entire processing procedure) and low throughput. Since NGS technology and scMS methods are highly complementary, the future integration of both approaches holds promise for achieving new breakthroughs in the field of single-cell proteomics.82 SCM can provide deep insights into the metabolic patterns of cancer cells and help identify potential therapeutic targets; however, the limited sample volume of individual cells requires significantly higher sensitivity than that of traditional metabolomics. Additionally, the issues of metabolite leakage and rapid metabolite conversion during processing urgently need to be addressed.83 Notably, the application of single-cell spatial transcriptomics can compensate for the spatial information missing in single-cell transcriptomics, helping us understand how viral infection reshapes liver tissue architecture and the tumour microenvironment, thereby providing new insights for developing targeted therapeutic strategies. Single-cell spatial transcriptomics can be typically divided into two types: imaging-based and sequencing-based approaches. The core limitation of imaging-based spatial transcriptomics lies in its low throughput, primarily stemming from time-consuming probe hybridisation and image acquisition processes, while the key constraint of sequencing-based spatial transcriptomics is insufficient resolution, which is limited by the density of surface probes.84-87 Through continuous optimisation of integration strategies between single-cell multi-omics and spatial technologies, and by overcoming existing bottlenecks such as resolution and throughput limitations, it is expected to achieve a comprehensive analysis of virus–host interaction dynamic networks during hepatitis-to-liver cancer transformation from a single-cell perspective, thereby laying the foundation for developing precise intervention strategies to block carcinogenic processes.
5.1.2 Construction of virus–host protein interaction networks: Revealing carcinogenic mechanisms and identifying novel therapeutic targets
The complex interaction networks between viral and host proteins are critical for understanding the transformation from viral hepatitis to HCC. These interactions not only influence the efficiency of viral replication and transmission but also may significantly impact host cell signalling, metabolic reprogramming and immune evasion, thereby facilitating the development and progression of HCC. In recent years, high-throughput screening techniques that integrate affinity purification, mass spectrometry analysis and bioinformatics have emerged as powerful tools for the systematic identification of virus–host protein interactions. For instance, a recent study employing APEX2 proximity labelling technology in conjunction with mass spectrometry analysis successfully identified host proteins associated with HBV core protein in living cells.88 Furthermore, time-resolved proteomics and phosphoproteomics analyses can elucidate the dynamic changes in host cell protein interaction networks following viral infection, thus enhancing our understanding of how viruses hijack host cellular mechanisms and induce carcinogenesis. Importantly, these interaction networks may display significant differences across various viral genotypes and host genetic backgrounds, thus necessitating validation in diverse patient populations. Future research should further investigate how virus–host protein interactions reshape the tumour microenvironment and regulate immune evasion, as this may yield important insights for developing novel immunotherapeutic strategies.
5.1.3 Deciphering the long-term impact of virus-induced epigenetic reprogramming on HCC initiation and progression
Virus-induced host epigenetic reprogramming represents a crucial mechanism in HCC development, encompassing primarily four aspects: alterations in DNA methylation patterns, abnormal histone modifications, chromatin structure remodelling and regulation of non-coding RNA expression. Recent studies suggest that these epigenetic alterations not only affect immediate gene expression but also potentially exert long-term effects on hepatocytes through epigenetic memory mechanisms, thus increasing the risk of HCC development. For instance, HBx has been demonstrated to silence the expression of tumour-suppressor genes by interacting with the DNA methyltransferase DNMT3A.89 Furthermore, viral infection can potentially alter global chromatin accessibility by inducing the expression of histone deacetylases (HDACs), consequently affecting the expression of multiple cancer-related genes. Notably, recent research has revealed that viral infection may contribute to regulating hepatocyte fate determination and malignant transformation by influencing novel epigenetic modifications, including DNA hydroxymethylation and histone methylation.90 Additionally, lncRNA plays crucial roles in virus-induced epigenetic reprogramming, as exemplified by HBx upregulating the oncogenic lncRNA HUR1, which promotes HCC growth by inhibiting p53.91 Future research should employ single-cell epigenomics technologies to decipher the dynamic changes in the epigenetic landscape of hepatocytes following viral infection, and explore therapeutic strategies targeting these epigenetic alterations, including the use of epigenetic modulators to reverse virus-induced oncogenic epigenetic changes.
5.2 Development of biomarkers for early diagnosis and prognosis prediction
Based on a comprehensive understanding of molecular mechanisms, the development of highly sensitive and specific biomarkers is crucial for the early diagnosis and personalised treatment of HCC. In recent years, the rapid advancement of multi-omics technologies, including genomics, transcriptomics, proteomics and metabolomics, has created new opportunities for the discovery of HCC biomarkers. These multidimensional biomarkers have the potential to not only improve diagnostic accuracy but also provide more comprehensive information for patient prognosis assessment and treatment decision-making.
5.2.1 Utilising liquid biopsy technology to develop non-invasive diagnostic methods based on circulating tumour DNA (ctDNA) and exosomes
Liquid biopsy technology has demonstrated significant promise in elucidating the mechanisms of HCC caused by viral hepatitis. This technology primarily detects biomarkers including ctDNA, circulating tumour cells (CTCs) and exosomes in the blood, enabling the identification of microscopic tumour lesions that are undetectable by routine clinical diagnosis, thereby providing new avenues for early diagnosis, treatment response monitoring and prognosis evaluation of HCC. Recent studies have demonstrated that methylation patterns and gene mutation spectra in ctDNA can accurately reflect the molecular characteristics of HCC,92 while miRNAs and lncRNAs in exosomes show promise as novel diagnostic markers.93 Furthermore, the integration of artificial intelligence and machine learning algorithms has the potential to further enhance the diagnostic accuracy and predictive capabilities of liquid biopsies.
5.2.2 Integrating multi-omics data to construct HCC risk prediction models
The integration of multi-omics data enables researchers to identify key molecular events associated with HCC occurrence and progression. HCC risk prediction models can be developed by integrating information from multiple sources (e.g., TCGA, ICGC databases), including RNA-seq, miRNA-seq, methylation data, proteomics data and clinical features. This process involves utilising advanced statistical and machine learning methods (such as random forests, support vector machines and deep learning) to screen for features associated with HCC risk, and subsequently combining clinical features (such as age, gender and medical history) with omics features. Furthermore, the incorporation of time series analysis and dynamic modelling techniques enhances the prediction of HCC progression. Large-scale, multi-centre prospective cohort studies facilitate the validation and optimisation of these models, resulting in more accurate and personalised HCC risk prediction tools. These refined models have the potential to not only enhance early screening efficacy but also inform the development of precision treatment strategies.
5.2.3 Investigating the relationship between viral integration patterns and HCC prognosis, and developing novel molecular classification methods
Hepatitis viruses, particularly HBV, frequently induce chromosomal instability and alter gene function and normal expression through DNA integration. The frequency of HBV integration in tumours is significantly higher than in adjacent normal liver tissue,94 and the integration frequency positively correlates with tumour size, degree of cirrhosis and α-fetoprotein levels,95 indicating that HBV integration serves as one of the indicators of poor prognosis in HCC patients. Recent studies have demonstrated that HBV integration not only impacts host gene expression but may also facilitate tumour progression through epigenetic modifications and regulation of lncRNA.31, 66 Furthermore, the implementation of high-throughput sequencing technologies has elucidated HBV integration hotspots and their influence on key cancer genes. By investigating the relationship between viral integration patterns and HCC prognosis, a more comprehensive molecular classification system can be developed to predict patient outcomes and inform treatment decisions. This integrated classification approach not only enhances the accuracy of prognosis prediction but also establishes a foundation for personalised selection of immunotherapy and targeted therapy. Additionally, novel molecular classification methods facilitate the elucidation of specific mechanisms of viral integration in HCC initiation and progression, offering a theoretical basis for developing innovative therapeutic strategies. In particular, the implementation of targeted therapies and gene editing techniques directed at specific viral integration patterns has paved the way for precision treatment of HCC.
5.3 Development of targeted prevention and treatment strategies
Based on the elucidated molecular mechanisms underlying the progression from viral hepatitis to HCC, more targeted prevention and treatment strategies are being developed. In recent years, with an enhanced understanding of the relationship between viral hepatitis and HCC, substantial progress has been made in developing prevention and treatment strategies. Nevertheless, numerous challenges persist, necessitating continued efforts to explore more efficacious approaches. In recent years, many studies have focused on exploring potential therapeutic targets for liver cancer caused by viral hepatitis (Table 1).
| Target | Rationale | Refs. |
|---|---|---|
| TNNT1 | In mouse experiments, knockdown or knockout of the TNNT1 gene can eliminate HBV-induced EMT and liver fibrosis. | 96 |
| ABHD16A | ABHD16A negatively regulates the anti-HBV activity of interferon-induced transmembrane protein 1. | 97 |
| MKI67 | MKI67 is significantly overexpressed in HBV/HCV-related HCC. | 98 |
| SQLE | In viral hepatitis-related HCC, SQLE expression is enhanced and associated with weakened anti-tumour immunity, potentially involving reduced NK cell and T cell infiltration, and increased monocyte and macrophage infiltration. | 99 |
| IDR of HBx protein | The IDR of the HBx protein is involved in regulating cccDNA transcription, viral replication and carcinogenesis. | 100 |
| DYRK4-K133 | DYRK4-K133 can inhibit HBV replication. | 101 |
| IGF2BP3–VEGFA axis | In HBV-related HCC, the IGF2BP3–VEGFA axis promotes angiogenesis. | 102 |
| The NS3-induced circ_0001175/miR-130a-5p/MDM4/P53 pathway | NS3 promotes HCV-related HCC development through the circ_0001175/miR-130a-5p/MDM4/P53 axis. | 103 |
| DTL | DTL is involved in regulating HBV-related HCC by affecting the cell cycle and mediating the formation of a positive feedback loop of HBx-DTL-CRL4s. | 104 |
| IL-35 | Inhibiting IL-35 expression can suppress the IL6-STAT3 signalling pathway, thereby effectively slowing the development of HBV-related HCC. | 105 |
| Pre-S mutants | Pre-S mutants can promote HBV-related HCC development by activating several oncogenic signalling pathways. | 106 |
| MicroRNA-3145 | MicroRNA-3145 inhibits viral replication by downregulating HBS and HBX. | 107 |
| NTCP | NTCP affects hepatocyte metabolism and facilitates HBV entry into hepatocytes. | 108 |
| ZWINT, MELK, DLGAP5, BIRC5, AURKA, HMMR, CDK1, TTK and MAD2L1 | ZWINT, MELK, DLGAP5, BIRC5, AURKA, HMMR, CDK1, TTK and MAD2L1 are significantly correlated with poor prognosis in patients with HBV-related HCC. | 109 |
| Tim-1 | Tim-1 promotes the development of hepatocellular carcinoma by influencing abnormal changes in the extracellular matrix. | 110 |
| lncRNA POLR2J4 | lncRNA POLR2J4 promotes cell proliferation, migration and invasion by regulating miR-214-3p. | 111 |
| ACSL4 | Inhibition of ACSL4 expression can upregulate farnesoid X receptor expression, reduce bile acids and weaken M2 macrophage polarisation, thereby alleviating the progression of HBV-related HCC. | 112 |
| RRM2 | Downregulation of RRM2 expression can reduce the deterioration of HBV-related HCC and accelerate apoptosis. | 113 |
| mTOR | HBV may achieve self-replication by activating the mTOR pathway. | 114 |
| ERK1/2 signalling pathway | HBx promotes the occurrence and progression of HCC by activating the ERK1/2 signalling pathway and promoting RSK2 expression. | 18 |
| RSK2 | RSK2, as an important cancer-related protein, its upregulated expression significantly promotes the occurrence and progression of HCC. | 18 |
| HBx/PRMT9/HSPA8/CD44 signalling axis | HBx promotes the occurrence and progression of HCC by inhibiting ferroptosis through the regulation of the HBx/PRMT9/HSPA8/CD44 signalling axis. | 19 |
| NF-κB signalling pathway | Through its interaction with NF-κB, HBx activates the NF-κB signalling pathway and promotes the expression of its downstream target genes, thereby contributing to the occurrence and progression of HCC. | 20 |
| GRP78–Smad4–MAN1B1 axis | HBx activates PI3K/mTOR signalling through the GRP78–Smad4–MAN1B1 axis, exacerbating HCC progression. | 21 |
| PI3K/Akt/Oct-1 signalling pathway | Ectopic expression of HBx significantly promotes HCC cell metastasis and colonisation by activating the PI3K/Akt/Oct-1 signalling pathway and enhancing RHAMM expression. | 22 |
| RAD51C | HBx can promote RAD51C expression, thereby promoting HBV replication. | 25 |
| SELENBP1 | HBx can lead to decreased SELENBP1 expression, which may be one of the important mechanisms by which HBV induces HCC. | 26 |
| miR-210-3p | miR-210-3p can inhibit PTPN18 expression and also upregulate HBx expression levels in HBV-infected Huh7/NTCP cells. | 27, 28 |
| miR-1236 | HBx can indirectly lead to AFP upregulation by downregulating miR-1236 expression. | 29 |
| miR-329 | HBx can indirectly lead to AFP upregulation by downregulating miR-329 expression. | 29 |
| miR-137 | HBx can promote the occurrence and progression of HCC by inhibiting the expression of tumour-suppressive miR-137. | 30 |
| lncRNA-ATB | HBx upregulates lncRNA-ATB expression, significantly promoting HCC metastasis and invasion. | 31 |
| PTEN | HBV can lead to decreased PTEN protein expression, subsequently activating the PI3K/AKT signalling pathway and promoting HCC occurrence and progression. | 32 |
| STC2 | Ectopic expression of HBx upregulates HMGA2 expression, thereby promoting STC2 expression and inhibiting apoptosis. | 34 |
| HMGA2 | Ectopic expression of HBx upregulates HMGA2 expression, thereby promoting STC2 expression and inhibiting apoptosis. | 34 |
| Acetylated AFP | HBx can increase the content of acetylated AFP; acetylated AFP can hinder the binding of PTEN to the pro-apoptotic protein caspase-3, thereby promoting HCC progression. | 35 |
| HMGB1 | HBx promotes VEGF expression by upregulating HMGB1 expression in HCC cells. | 36, 37 |
| SHBs | SHBs-induced ER stress can promote VEGFA expression and secretion and induce EMT in HCC cells. | 39, 40 |
| NAC1 | HBV can also enhance the suppression of host immune responses by upregulating NAC1 expression, thereby inhibiting the activation and proliferation of CD8+ T cells. | 43 |
| ISX | HCV core protein promotes metabolic disorders in HCV-infected cells by inducing ISX expression. | 45 |
| PIAS1 | HCV promotes lipid accumulation, creating a favourable environment for viral replication, by upregulating PIAS1 expression, which in turn affects microtubules and Septin 9. | 50 |
| Histone H3K4me3 | In HBV-related HCC, H3K4me3 levels are significantly elevated, and this increase in epigenetic modification is closely related to tumour occurrence and progression. | 66 |
| Histone H2A | HCV can downregulate the expression of the HOX gene family by reducing the monoubiquitination level of histone H2A. Abnormal expression of HOX gene products is closely related to tumourigenesis. | 67 |
| HOX | Abnormal expression of HOX gene products is closely related to tumourigenesis. | 67 |
| Smc5/6 complex | HBx can further promote the accumulation of DNA damage by degrading the Smc5/6 complex, which has an inhibitory effect on viral replication in human hepatocytes. | 72 |
- Abbreviations: ABHD16A, α/β-hydrolase domain-containing 16A; ACSL4, acyl-CoA synthetase long-chain family member 4; DTL, denticleless e3 ubiquitin protein ligase homolog; DYRK4, dual-specificity tyrosine-regulated kinase 4; EMT, epithelial–mesenchymal transition; ERK, extracellularly regulated protein kinases; H3K4me3, H3 lysine 4 trimethylation; HBc, HBV core protein; HBS, hepatitis B virus S; HBV, hepatitis B virus; HBX, hepatitis B virus X; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HMGB1, high-mobility group box 1; HOX, homeobox; IDR, intrinsically disordered region; ISX, intestine-specific homeobox; lncRNA-ATB, long non-coding RNA activated by transforming growth factor (TGF)-β; mTOR, mammalian target of rapamycin; NAC1, nucleus accumbens-associated protein 1; NGS, next-generation sequencing; NS3, non-structural protein 3; NTCP, sodium taurocholate co-transporting polypeptide; PIAS1, protein inhibitor of activated STAT1; PTEN, phosphatase and tensin homolog; RRM2, M2 subunit of ribonucleotide-diphosphate reductase; RSK2, ribosomal S6 kinase 2; SELENBP1, selenium binding protein 1; SQLE, squalene epoxidase; Tim-1, T-cell immunoglobulin and mucin domain 1; TNNT1, troponin T1; VEGF, vascular endothelial growth factor.
5.3.1 Limitations of current treatment approaches
The pronounced temporal and spatial heterogeneity exhibited by HCC presents substantial challenges in developing and implementing precise treatment strategies. The initiation and progression of HCC involve alterations in multiple signalling pathways, with cancer cells harbouring numerous mutations, including multiple driver genes. Consequently, single targeted therapies often fail to achieve optimal therapeutic efficacy, and due to factors such as genetic mutations, patients may exhibit varying degrees of reduced drug sensitivity, with some developing resistance. Furthermore, antiviral treatments have substantial limitations due to the emergence of drug resistance and the persistent carcinogenic effects of integrated viral DNA, even following viral eradication. Moreover, the complexity of the tumour microenvironment and its immunosuppressive effects are critical factors influencing treatment outcomes. Recent studies have demonstrated that HCC cells can shape a microenvironment conducive to tumour survival and growth by secreting specific cytokines and metabolites,115 further complicating therapeutic interventions.
5.3.2 Designing gene editing therapeutic strategies targeting viral integration hotspots
Based on the molecular mechanisms of viral integration, future therapeutic strategies can be developed to target these integration hotspots using gene editing approaches. Specifically, advanced gene editing technologies, such as CRISPR-Cas9, can be utilised to achieve precise targeting and excision of integrated viral DNA, thereby effectively eliminating its adverse effects on host gene expression. This therapeutic approach not only holds promise for providing new treatment options for patients already diagnosed with HCC but may also have the potential to prevent HCC progression in high-risk populations. Recent research advances have demonstrated that optimising the delivery methods of the CRISPR-Cas9 system, such as employing lipid nanoparticles or adeno-associated viral vectors, can significantly enhance the efficiency and specificity of gene editing.116, 117 Furthermore, the emergence of novel gene editing tools, such as Base Editors and Prime Editors, has opened up new possibilities for more precise and safer gene editing therapies.118
5.3.3 Development of small molecule inhibitors targeting virus–host protein interactions
The development of small molecule inhibitors that target virus–host protein interactions offers high specificity, potentially minimising harm to non-target cells or tissues. These inhibitors typically have low molecular weights, which makes them easier to synthesise and optimise, thus facilitating drug development and clinical application. Recent advances in structural biology and computer-aided drug design have opened up new opportunities in this field. By employing high-throughput screening and artificial intelligence algorithms, researchers have successfully developed several candidate small molecule inhibitors that target HBV–host interactions. For instance, nitazoxanide, a small molecule compound that targets the HBx–DDB1 protein interaction, has demonstrated promising antiviral activity.119
5.3.4 Investigation of combination strategies using immune checkpoint inhibitors and antiviral therapies
Immune checkpoint inhibitors enhance the immune system's ability to target cancer cells by blocking key regulatory pathways.120-123 Antiviral drugs, in contrast, eliminate viruses by inhibiting viral replication or preventing virus–host cell binding. The combination of these two approaches can achieve a more comprehensive therapeutic effect and potentially mitigate the development of drug resistance associated with monotherapy. This multi-modal combination therapy strategy shows promise in improving treatment outcomes by simultaneously inhibiting viral replication and enhancing anti-tumour immune responses. However, optimising the timing and dosage of combination therapies, as well as managing potential side effects, remains an important issue requiring further investigation.
5.4 Elucidating molecular and cellular heterogeneity in virus-related HCC
In-depth investigation of molecular heterogeneity in HBV- and HCV-related HCC, encompassing differences at genomic, transcriptomic and proteomic levels, provides a solid theoretical foundation and practical basis for developing precision medicine strategies.
5.4.1 Deciphering spatiotemporal heterogeneity of the HCC tumour microenvironment using high-resolution spatial transcriptomics
High-resolution spatial transcriptomics technologies, such as spatial transcriptomics and in situ sequencing, enable the acquisition of single-cell level gene expression data while preserving precise spatial information of cells within tissues, thus revealing the spatial distribution and functional heterogeneity of different cell populations in the HCC tumour microenvironment. The application of these advanced spatial transcriptomics technologies to investigate the heterogeneity of the HCC tumour microenvironment can elucidate the precise spatial distribution and interaction networks of various cell types within the tumour (such as tumour cells, immune cells, vascular endothelial cells and stromal cells). This high-resolution spatial heterogeneity analysis is crucial for gaining an in-depth understanding of HCC biological behaviour, elucidating dynamic changes in the tumour immune microenvironment, and developing precision therapeutic strategies targeting the tumour microenvironment.
5.4.2 Deciphering the complex relationships among viral integration, epigenetic modifications and genomic instability and their impact on HCC clonal evolution
An in-depth analysis of the complex relationships between viral factors (such as HBV genome integration and HCV-induced oxidative stress) and host genomic instability, as well as their mechanisms of influence on HCC clonal evolution and heterogeneity formation, can help identify key driving events and potential therapeutic targets, thereby facilitating the design of more individualised and precise treatment strategies for patients. For instance, in HCC patients with HBV or HCV infection, personalised treatment strategies can be developed based on multidimensional factors such as viral load, viral integration sites, degree of genomic instability (e.g., chromosomal instability index and microsatellite instability status) and tumour heterogeneity, potentially leading to improved patient outcomes. These personalised approaches may involve combination therapies utilising novel antiviral drugs (such as HBV functional cure agents), immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors), epigenetic modulators or other molecular targeted therapies, tailored to the specific characteristics of each patient's tumour. Furthermore, by systematically studying the dynamic impact of viral factors and genomic instability on HCC clonal evolution, and integrating multi-omics data (such as genomics, transcriptomics, proteomics and metabolomics) with artificial intelligence algorithms, more accurate models for predicting treatment response and prognosis can be developed. These advanced models would provide crucial support for achieving precision medicine and personalised management of HCC, ultimately improving patient care and outcomes.
5.4.3 Deciphering the association between different viral genotypes/subtypes and HCC molecular subtypes and prognosis
HCC has been categorised into various molecular subtypes, primarily including the proliferation class and non-proliferation class. In recent years, based on integrative analyses of multi-omics data, researchers have proposed more refined molecular classification systems, including iCluster, IMbased and CTNNB1 classification methods. These classifications not only reflect distinct molecular characteristics but are also closely associated with specific gene mutation spectra, signalling pathway activation patterns and clinical prognoses. Both HBV (genotypes A–J) and HCV encompass multiple genotypes and subtypes. Different viral genotypes/subtypes may lead to distinct molecular characteristics and epigenetic modification patterns in infected cells through specific virus–host interactions, immune evasion mechanisms and oncogenic pathways. In-depth investigation of the associations between different viral genotypes/subtypes and HCC molecular subtypes can help elucidate the molecular mechanisms underlying virus-induced HCC development and progression, thereby providing crucial clues for the development of antiviral drugs targeting specific viral subtypes and HCC-targeted therapeutics. Furthermore, such association studies may help establish more accurate prognostic prediction models and personalised treatment decision support systems, ultimately optimising clinical management strategies for HCC patients.
5.5 Developing novel experimental models and innovative research methods: Integrating multidisciplinary technologies to advance HCC research
The development of experimental models and innovative research methods that more accurately simulate human disease processes, while integrating multi-omics data and advanced technologies, facilitates rapid translation from basic research to clinical applications, thereby improving the prevention, diagnosis and treatment of HCC.
5.5.1 Utilising humanised mouse models to comprehensively study the dynamic process from viral infection to HCC development: From molecular mechanisms to immune microenvironment
Traditional mouse models demonstrate significant species differences in simulating human diseases, consequently certain viruses (such as HBV and HCV) are unable to directly infect mice or induce disease symptoms similar to those in humans, thus severely limiting in-depth research on the mechanisms of viral hepatitis-related HCC development. Humanised mouse models reconstruct the human immune environment through the transplantation of human cells or tissues (such as hepatocytes, immune cells or haematopoietic stem cells) into immunodeficient mice. These models are capable of simulating human-specific immune responses and disease progression processes, including multi-stage processes such as viral infection, chronic inflammatory response, liver fibrosis, cirrhosis, cell proliferation and eventual carcinogenesis, thereby significantly improving the accuracy and clinical relevance of disease simulation. At present, humanised mouse models have been developed that successfully simulate the process of chronic hepatitis and HCC development caused by HBV infection,124 thus providing valuable tools for studying virus–host interactions.
From a drug development perspective, conducting drug screening and efficacy evaluation using humanised mouse models can significantly accelerate the drug development process and improve the effectiveness and safety of drugs. Moreover, these models provide an ideal platform for evaluating combination treatment strategies, such as antiviral therapy in conjunction with immune checkpoint inhibitors. By systematically studying the associations between different viral subtypes (such as HBV genotypes A–J and HCV genotypes 1–6) and molecular characteristics of HCC, humanised mouse models can provide a robust theoretical foundation for personalised treatment. This research approach facilitates the identification of cancer driver genes and signalling pathways associated with specific viral subtypes, thereby providing crucial evidence for the development of targeted therapeutic strategies.
Research findings from humanised mouse models can provide essential evidence for clinical translational studies. By conducting systematic comparative analyses between model research results and large-scale clinical data, the accuracy and reliability of the models can be comprehensively validated, thus accelerating the translation of research findings into clinical applications. Recent studies have demonstrated that humanised mouse models exhibit high clinical relevance in predicting the efficacy of antiviral drugs and immunotherapies, offering valuable guidance for optimising clinical trial designs and developing personalised treatment plans.125, 126
5.5.2 Development of liver organoid models for viral hepatitis: Integrating 3D culture techniques and microfluidic systems to simulate complex liver microenvironments for high-throughput drug screening and precision personalised therapy
Liver organoid models associated with viral hepatitis can accurately simulate the structural and functional abnormalities of the liver caused by viral infection, thus more precisely reflecting the pathophysiological processes of human viral hepatitis. These advanced three-dimensional (3D) models not only simulate the early stages of viral infection but also recapitulate the processes of liver fibrosis and carcinogenesis caused by long-term infection, offering an ideal platform for studying the mechanisms of viral hepatitis progression to HCC. These highly complex models incorporate various liver cell types, including hepatocytes, cholangiocytes, HSCs, Kupffer cells and diverse immune cells, facilitating accurate simulation of the dynamic intercellular interactions and complex immune response processes following viral infection. Recently, multicellular liver organoids for studying HCV infection have been developed, yielding unprecedented insights into virus–host interactions.127
Based on individual patient characteristics (including genetic background, epigenetic modifications, viral infection subtypes, disease stages and treatment history), highly personalised models of viral hepatitis can be developed. These ‘patient-derived’ organoid models serve as powerful tools for achieving precision medicine, allowing researchers to predict individual patient responses to different treatment regimens in vitro, thus optimising therapeutic strategies. Through systematic analysis of these highly personalised models' responses to various candidate drugs, researchers can develop precise individualised treatment plans for patients, potentially improving therapeutic efficacy and long-term survival rates significantly. Recent studies have demonstrated that this approach has the potential to predict patient responses to immune checkpoint inhibitors and targeted therapies, thereby guiding the development of combination treatment strategies.128
5.5.3 Application of cutting-edge artificial intelligence technologies: Integrating multi-omics big data and clinical information to develop precise prediction models, assess HCC risk and optimise individualised treatment strategies
Advanced artificial intelligence technologies, particularly deep learning and ensemble learning algorithms, have the capability to reveal non-linear relationships and multi-level interactions that are challenging to capture with traditional statistical methods through complex data mining and pattern recognition.129 This approach provides researchers with novel perspectives and robust analytical tools, facilitating a deeper understanding of HCC pathogenesis, progression and heterogeneity. Recent studies have demonstrated that deep learning models based on convolutional neural networks exhibit superior performance in analysing liver imaging data, leading to significant improvements in diagnostic accuracy.130
Multidimensional data analysis, which integrates massive data from various sources including genomics, transcriptomics, epigenomics, proteomics, metabolomics, radiomics and clinical phenotype data,131, 132 enables artificial intelligence technologies to comprehensively reveal the multi-level molecular mechanisms and complex regulatory networks underlying HCC development and progression. This systematic analytical approach not only facilitates the discovery of novel therapeutic targets and prognostic biomarkers, but also enables the construction of precise risk prediction models. Recent studies utilising multi-omics integrated analysis and machine learning algorithms have successfully identified novel molecular markers associated with the risk of HBV-related HCC development, thereby providing new therapeutic targets for HBV-related HCC and offering new strategies for early screening and intervention.133
AUTHOR CONTRIBUTIONS
Writing–original draft: Keyin Zheng, Aimin Jiang, Zhengrui Li, Li Chen, Kailai Li, Junyi Shen, Hank Z. H. Wong, Quan Cheng, Jian Zhang, Anqi Lin, Peng Luo. Conceptualisation: Jian Zhang, Anqi Lin, Peng Luo. Investigation: Keyin Zheng, Aimin Jiang, Zhengrui Li, Li Chen, Kailai Li, Junyi Shen, Hank Z. H. Wong, Quan Cheng. Writing–review and editing: Keyin Zheng, Aimin Jiang, Zhengrui Li, Li Chen, Kailai Li, Junyi Shen, Hank Z. H. Wong, Quan Cheng, Jian Zhang, Anqi Lin, Peng Luo. Visualisation: Keyin Zheng, Aimin Jiang, Zhengrui Li. All the authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The authors have nothing to report.
CONSENT FOR PUBLICATION
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




