Complex, cell-type-specific role of EEPD1: Bridging TNF-α, inflammation and apoptosis in endothelial cells
Atherosclerotic cardiovascular disease (ASCVD) is one of the leading causes of mortality and morbidity worldwide. Plasma low-density lipoprotein (LDL) cholesterol levels are positively correlated with the risk of ASCVD. Current lipid-lowering medications, such as statins and PCSK9 inhibitors, can effectively reduce plasma LDL cholesterol levels and lower ASCVD risk.1-3 However, the residual risk remains high.4 Therefore, there is an urgent need to develop alternative therapeutic strategies for patients who do not respond to existing treatments.
Atherosclerosis is the hardening and narrowing of arteries, accompanied by chronic inflammation. The development and progression of atherosclerosis involve the dysfunction and activation of multiple cell types and signalling pathways within the arteries. Emerging evidence demonstrates that vascular endothelial cells play a critical role in this process, highlighting their potential as a therapeutic target for preventing and treating ASCVD.
Endothelial cells line the inner surface of blood vessels and are essential for regulating vascular tone and structure. They control the permeability of blood vessels, preventing the entry of lipoproteins and leukocytes and thereby reducing the development of atherosclerosis. Additionally, endothelial cells have antiplatelet, anticoagulant and fibrinolytic functions. However, when endothelial cells become activated and dysfunctional, they promote atherosclerosis progression and contribute to cardiovascular events, such as plaque erosion. Activated endothelial cells express adhesive molecules and inflammatory mediators, which stimulate inflammation. Dysfunctional endothelial cells compromise vascular integrity and increase the permeability of lipoprotein particles, leading to lipid accumulation in the intima.5 Therefore, targeting endothelial cells presents a promising therapeutic approach for the treatment of ASCVD. However, achieving this requires a comprehensive understanding of the pathophysiology and underlying mechanisms of endothelial cells in atherogenesis, areas that remain elusive.
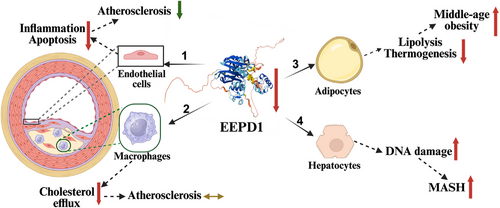
In a recent publication in Clinical and Translational Medicine, Yu et al. reported that Endonuclease/Exonuclease/Phosphatase Family Domain Containing 1 (EEPD1) is a crucial regulator of inflammation and apoptosis in endothelial cells during atherogenesis, acting through the Kruppel-like factor 4 (KLF4)–EEPD1–extracellular signal-regulated kinase (ERK) signalling axis (Figure 1).6 EEPD1, an endonuclease, plays a vital role in DNA repair and maintaining genome stability.7 The authors reported that EEPD1 expression was elevated in both human and murine atherosclerotic plaques. They demonstrated that knockout of Eepd1 in ApoE-deficient mice provided significant vascular protection, reducing macrophage infiltration, endothelial cell apoptosis, inflammation and plaque size. In contrast, overexpression of EEPD1 exacerbated the progression of atherosclerosis. Mechanistically, their research revealed that EEPD1 promoted ERK phosphorylation, which led to decreased expression of BCL2 (an inhibitor of apoptosis) and increased expression of vascular cell adhesion molecule 1(VCAM-1) and intercellular adhesion molecule 1 (ICAM-1), as well as monocyte chemoattractant protein 1 (MCP-1). These changes enhanced inflammation and apoptosis in endothelial cells, worsening atherosclerosis. Furthermore, the authors found that KLF4 inhibited EEPD1 expression, and that inhibiting KLF4 abolished the atheroprotective effects associated with EEPD1 deficiency. These findings underscore the therapeutic potential of inhibiting endothelial EEPD1 in ASCVD.
Interestingly, EEPD1 has been reported to be upregulated by the liver X receptor, which enhances cholesterol efflux in macrophages.8 Given the critical role of macrophage cholesterol efflux in reverse cholesterol transport, one might expect that the absence of macrophage EEPD1 would worsen atherosclerosis. However, a study by van Wouw showed that transplanting bone marrow lacking Eepd1 into Ldlr−/− knockout mice did not affect atherosclerosis development compared to mice transplanted with wild-type bone marrow, despite bone marrow-derived macrophages with EEPD1 deficiency showing reduced cholesterol efflux upon LXR activation (Figure 1).9 It would be interesting to investigate whether EEPD1 also promotes inflammation and apoptosis in macrophages, potentially offsetting its protective effect of enhanced cholesterol efflux.
EEPD1 is also highly expressed in adipocytes. Whole-body knockout of Eepd1 (Eepd1−/−) showed no notable effect on body weight gain in mice fed a regular chow diet for up to 4 months. However, Eepd1−/− mice displayed increased body weight gain when fed a high-fat or Western-type diet.9 Knockout of adipocyte Eepd1 also did not significantly affect body weight gain in mice on a regular chow diet for up to 4 months, but adipocyte Eepd1 knockout mice showed substantial weight gain after 8–10 months, indicating obesity during middle age. Consistently, EEPD1 expression was reduced in obese individuals, and restoring EEPD1 effectively mitigated obesity by promoting adipose lipolysis and thermogenesis.10 Therefore, enhancing rather than inhibiting adipocyte EEPD1 may be beneficial for addressing obesity. Similarly, in hepatocytes, promoting EEPD1 degradation exacerbated the progression of metabolic dysfunction-associated steatohepatitis (MASH), while suppressing its degradation mitigated the issue.11 These findings reveal the complex, cell-type-dependent roles of EEPD1 and underscore the necessity for precise, cell-type-specific regulation of EEPD1 activity to avoid off-target effects in therapy (Figure 1).
In conclusion, by utilising a comprehensive in vivo and in vitro approach that includes genetic models, high-throughput transcriptomics, histological analyses and mechanistic assays, the authors present compelling evidence that unmasks a novel role of EEPD1 in cardiovascular pathology. Notably, while EEPD1 is well-known for its functions in DNA damage repair and tumourigenesis, this research identifies it as a crucial mediator of endothelial inflammation and apoptosis in the context of atherosclerosis. Its dual involvement in oncogenesis and vascular inflammation holds significant promise for patients facing both cancer and cardiovascular disease. Given the complexity of EEPD1's roles and expression patterns, future studies should employ cell-type-specific EEPD1 knockout mice to accurately delineate its functions.
ACKNOWLEDGEMENTS
Da-Wei Zhang is supported by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2024-06088). Rong Li is supported by funding from Children's Hospital of Chongqing Medical University, China.




