Friend or foe: The paradoxical roles of cancer-associated fibroblasts in tumour immunotherapy
Minying Xiong, Aimin Jiang and Zhengrui Li are joint authors, contributed equally to this work and share first authorship.
Abstract
Cancer-associated fibroblasts (CAFs) represent critical cellular components of the tumor microenvironment and have garnered widespread attention in the field of tumor immunology. However, given the pronounced heterogeneity of CAFs, research investigating their impact on tumor immunity has yielded diverse and often contradictory results. Therefore, in this review, we have systematically summarized previous studies to comprehensively elucidate the role of CAFs in the tumor immune microenvironment and have explored the bidirectional regulatory effects of CAFs on immune cells and immune molecules within this complex niche. We highlight the multifaceted role of CAFs in cancer immunotherapy, focusing on their impact on immunotherapeutic efficacy, as well as the synergistic effects between CAF-targeted therapies and immunotherapies in anti-cancer treatment. Addressing the heterogeneity of CAFs, we also critically analyze controversies surrounding these cells in the field of tumor immunology and propose strategic directions for future investigations targeting this cell population. Our comprehensive analysis provides a strategic framework for future research directions and clinical translation of CAF-targeted strategies, ultimately facilitating the development of more effective and personalized cancer immunotherapeutic approaches.
Cancer remains a formidable challenge that modern medicine has still not fully overcome. The tumour immune microenvironment (TIME), which comprises an intricate network of immune cells and molecules surrounding tumour cells, significantly influences tumour progression and invasion, thereby emerging as a potential target for cancer therapy.1, 2 Cancer-associated fibroblasts (CAFs), functioning as crucial cellular components of the tumour microenvironment (TME), play pivotal roles in multiple aspects of cancer biology, including tumorigenesis, immune suppression, therapeutic resistance, and extracellular matrix (ECM) remodelling.2, 3 Immunotherapy has emerged as a critical strategy for cancer treatment, primarily encompassing immune checkpoint inhibitors (ICIs), adoptive cell therapy (ACT), cancer vaccines, and oncolytic virus therapy.4 In recent years, the complex interactions between CAFs, TIME, and immunotherapy have increasingly become a focal point of intensive scientific investigation. This review systematically summarizes current research progress regarding CAFs in TIME and cancer immunotherapy, and critically discusses existing controversies and potential future research directions, thereby providing novel insights for CAF-based therapeutic strategies and clinical translation.
Recent research has demonstrated that CAFs represent a highly heterogeneous population with multiple subtypes that researchers have identified.1 Numerous studies have summarized the dual characteristics of CAFs in both promoting and inhibiting cancer1, 4, 5 (Figure 1), although their tumour-promoting properties have been extensively documented. CAFs remodel the ECM to form dense structures that simultaneously inhibit immune cell infiltration while enhancing tumour cell metastasis and facilitating immune escape.1, 4 CAFs can increase the number of tumour-associated macrophages (TAMs) and regulate macrophage differentiation toward the protumorigenic (M2) phenotype1, 3, 4; CAFs also influence the polarization of tumour-associated neutrophils (TANs) and promote the activation of N2 phenotype, thereby inducing immunosuppressive effects within the TME. Moreover, the function and maturation of dendritic cells, mast cells (MCs), natural killer (NK) cells, and myeloid-derived suppressor cells (MDSCs) are all modulated by CAFs, collectively contributing to an immunosuppressive microenvironment. Notably, some of the aforementioned immune cells can reciprocally enhance the tumour-promoting functions of CAFs, establishing a positive feedback loop.1, 3, 4 With respect to adaptive immunity, inflammatory CAFs (iCAFs) induce dysfunction of CD8+ and CD4+ T cells and promote the activation of immunosuppressive cells—particularly by promoting T helper cell 2 (Th2) responses while inhibiting T helper cell 1 (Th1) responses. Furthermore, iCAFs facilitate the conversion of effector T cells into regulatory T cells (Tregs) and enhance their infiltration.1, 4 At the molecular level, CAFs indirectly promote tumour malignant transformation and invasion through multiple pathways, including 1) the expression of immune checkpoint ligands such as programmed cell death ligand 1 (PD-L1), programmed cell death ligand 2 (PD-L2) and B7-H3/H4; 2) the secretion of chemokines and cytokines including transforming growth factor-β (TGF-β), interleukin-6 (IL-6), C-C motif chemokine ligand 2 and C-X-C motif chemokine 12 (CXCL12); 3) the production of exosomes.1, 3, 5-9
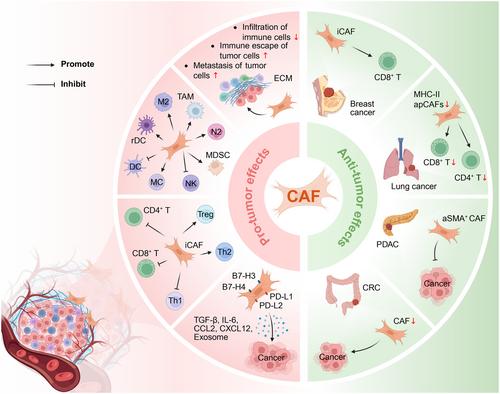
Although CAFs predominantly exert carcinogenic effects within the TIME, certain CAF subtypes have demonstrated the capacity to inhibit tumour growth. In colorectal cancer (CRC), CAFs predominantly exhibit a tumour-promoting role; however, their complete depletion paradoxically leads to significantly increased tumour cell invasiveness.9 In lung cancer mouse models, depletion of MHC-II-expressing antigen-presenting CAFs results in a significant reduction in CD4+ and CD8+ T cell infiltration. In breast cancer tissues, the abundance of specific iCAF subpopulations exhibits a positive correlation with CD8+ T cell infiltration.1 The α-smooth muscle actin (αSMA)-positive CAFs exhibit tumour-restricting functions in pancreatic ductal adenocarcinoma (PDAC).7 Collectively, these findings indicate that specific CAF subpopulations play a critical role in maintaining anti-tumour immune responses under particular conditions.
Numerous studies have demonstrated that CAFs are closely associated with immunotherapy efficacy (Figure 2). CAFs have been conclusively shown to influence the response to programmed cell death protein 1 (PD-1)/PD-L1 inhibitor immunotherapy in various solid tumours, including non-small cell lung cancer, oesophagal squamous cell carcinoma, CRC, and breast cancer.4 As previously mentioned, CAFs can promote TAM generation, which further exacerbates tumour immunosuppression and subsequently leads to resistance to PD-1/PD-L1 blockade therapy.4 In pancreatic adenocarcinoma, the ECM protein tenascin-C (TNC) is primarily produced and expressed by CAFs, and high TNC expression has been significantly associated with poor response to ICI therapy in patients. Therefore, strategies targeting CAF-derived TNC or other matrix components have been proposed as potentially beneficial approaches for enhancing immunotherapy efficacy.1 Emerging research has established that TGF-β-activated CAF subpopulations in fibrotic microenvironments are significantly associated with poor efficacy of anti-PD-L1 ICI therapy.6 A growing body of studies has demonstrated that CAFs induce immunosuppressive microenvironments through multiple mechanisms, thereby compromising immunotherapy outcomes. These specific mechanisms include: (1) upregulation of PD-1 and cytotoxic T-lymphocyte-associated protein 4 expression on Tregs; (2) inhibition of CD8+ T cell infiltration and attenuation of their cytotoxic function; (3) production of exosomes containing immunosuppressive molecules; (4) induction of macrophage migration inhibitory factor (MIF) expression.4, 10
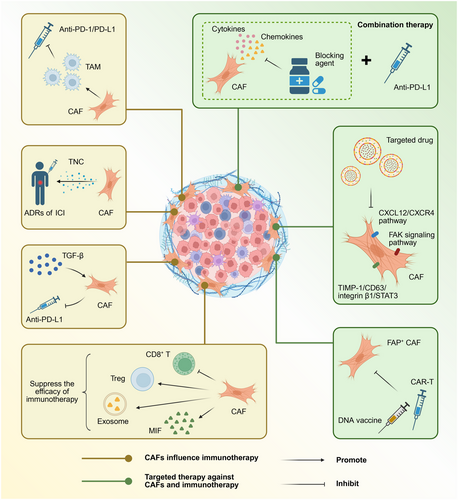
In recent years, an increasing body of studies has begun to explore the potential enhancing effects of CAF-targeting drugs on anti-cancer immune responses. Therapeutic approaches targeting CAFs primarily encompass three major aspects: inducing CAF depletion, inhibiting CAF activation and function, and limiting CAF-induced ECM remodelling.3 Elimination strategies targeting fibroblast activation protein (FAP)-positive CAFs, including DNA vaccination and chimeric antigen receptor T (CAR-T) cell therapy, have emerged as critical complementary approaches for enhancing the efficacy of other immunotherapeutic strategies.2, 3 Multiple preclinical studies have explored the therapeutic efficacy of FAP inhibitors targeting FAP-positive CAFs across various tumour models.1 Additionally, targeting the CXCL12/CXCR4 pathway, focal adhesion kinase signalling pathway, and TIMP-1/CD63/integrin β1/STAT3 signalling loop in CAFs has been identified as a series of highly promising therapeutic approaches.3, 5 Combination therapeutic strategies involving CAF-derived cytokine and chemokine blockers with immunotherapies (including anti-PD-L1 immunotherapy) are currently undergoing systematic investigation.2
As previously discussed, although CAFs predominantly exhibit tumour-promoting effects within the immune microenvironment, emerging research has revealed that specific CAF subpopulations can actually inhibit tumour progression.1, 5 For instance, in various PDAC mouse models, depletion of αSMA+ CAFs paradoxically accelerates tumour invasion, resulting in decreased overall survival, whereas depletion of FAP+ CAFs significantly inhibits tumour growth. Furthermore, conditional IL-6 knockout or collagen type I (Col1) depletion in αSMA+ CAFs exhibits completely opposite effects, whereas conditional depletion of Col1 in fibroblast-specific protein 1-positive CAFs elicits no significant effect on tumour cells.1 These contradictory findings underscore the considerable complexity of CAF-targeted therapies, highlighting the critical need for researchers to characterize more precisely the biological functions of distinct CAF subpopulations and their respective roles in the TME. Additionally, targeting the FAP gene through immunotherapeutic approaches to attenuate FAP+ CAF infiltration has been considered a valuable complementary strategy for enhancing the efficacy of existing immunotherapeutic modalities. However, it is noteworthy that bone marrow mesenchymal stem cells and skeletal muscle tissues also express FAP, rendering these tissues susceptible to attack and destruction by FAP-reactive CAR-T cells, potentially resulting in severe lethal toxicity and cachexia. This adverse reaction may be attributed to insufficient specificity of the single-chain variable fragment incorporated within the CAR construct; therefore, while FAP-targeted therapeutic research necessitates a more cautious approach, this direction continues to offer significant potential for translational clinical research.2
To better understand the crosstalk between CAFs and the TIME, a comprehensive investigation is necessary to elucidate the detailed mechanisms of interaction between various immune cells (such as TANs, TAMs, MCs, NK cells and T lymphocytes) and CAFs.3, 4 The molecular mechanisms and signalling pathways through which CAFs induce immune checkpoint molecule expression also require further elucidation.3 The development of accurate, stable, and highly specific detection methods for CAF subtypes constitutes a critical direction for future in-depth research. Targeting CAFs is considered an effective strategy to enhance tumour immunotherapy efficacy; however, additional research is essential to systematically characterize CAF heterogeneity, functional differences, and specific markers.1, 2, 5 Given that the dual-action mechanisms of CAFs in tumour progression still require in-depth exploration, targeting specific ECM components may serve as a temporary transitional approach for CAF-targeted therapy, thus providing a feasible alternative direction for cancer treatment.1 In the future, large-scale multicenter prospective randomized clinical trials will be necessary to evaluate the long-term efficacy and safety of CAF-targeted therapeutic strategies and to assess their applicability across diverse tumour types.3
In conclusion, CAFs represent highly promising therapeutic targets for malignancies, and a comprehensive analysis of the multidimensional mechanisms by which CAFs influence the TIME and modulate responses to immunotherapy significantly contributes to the development of more precise and personalized cancer treatment approaches. Continued in-depth research on CAF-targeted therapy and its subsequent translation into clinical applications holds promise for enhancing immunotherapy efficacy, overcoming treatment resistance mechanisms, and providing novel therapeutic modalities for comprehensive cancer management.
AUTHOR CONTRIBUTIONS
Writing-original draft, Minying Xiong; Conceptualization, Jian Zhang, Anqi Lin, Suyin Feng and Peng Luo; Investigation, Minying Xiong, Aimin Jiang, Zhengrui Li, Jian Zhang, Anqi Lin, Suyin Feng and Peng Luo; Writing-review and editing, Minying Xiong, Aimin Jiang, Zhengrui Li, Hank Z. H. Wong, Jian Zhang, Anqi Lin, Suyin Feng and Peng Luo; Visualization, Minying Xiong. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
Not applicable.
ETHICS STATEMENT
Not applicable.




