Structural insights into retinoic acid receptor activation and selective modulators
Yining Song and Wenrui Zhao are co-first authors of this paper.
Abstract
Retinoic acid receptors (RARs), including RARα, RARβ and RARγ, serve as essential nuclear receptors that act as transcription factors activated by ligands. They predominantly regulate gene expression and affect various biological processes, including differentiation. Their dysregulation is implicated in various cancers and other diseases, notably acute promyelocytic leukaemia (APL), where the promyelocytic leukemia (PML)‒RARα fusion protein disrupts normal granulocyte maturation. All-trans retinoic acid, which promotes the degradation of this fusion protein is a key therapeutic agent for APL and is also involved in the treatment of other diseases. Recently, various selective RAR modulators targeting specific RAR subtypes have been developed, which show promise in treating cancer and other diseases. The structural biology of RARs reveals how ligand binding induces conformational changes that facilitate co-activator recruitment, thereby modulating transcription. This review explores the crystal structures of RARs in various activation states, detailing RARs’ interactions with retinoid X receptors, ligands, DNA and co-regulators, and emphasises the importance of understanding these mechanisms for the rational design of new RAR-targeted therapies. The potential for developing selective RAR modulators is highlighted, along with the need for comprehensive structural data to enhance our understanding of RAR functions in disease contexts. Future research directions include utilising advanced imaging techniques and artificial intelligence-driven predictions to elucidate the dynamics of RAR complexes, ultimately aiming to translate structural insights into clinical applications for various diseases.
1 INTRODUCTION
Retinoic acid receptors (RARs: RARα, RARβ and RARγ) are nuclear receptors that act as transcription factors activated by ligands when they bind with retinoic acid, a vitamin A derivative. RAR partners with retinoid X receptors (RXRs) to form a heterodimer complex in vivo, which governs gene expression and plays a role in significant biological processes, notably differentiation. These receptors are implicated in various diseases, with cancer being the most closely associated. Numerous studies provide evidence indicating that RARs play a role in tumourigenesis, recurrence and treatment. In cases of acute promyelocytic leukaemia (APL), the expression of the PML‒RARα fusion protein, which arises from a translocation involving chromosomes 15 and 17, interferes with the normal functionality of wild-type PML, thereby inhibiting the maturation of granulocytes.1 All-trans retinoic acid (ATRA), which stimulates RARα to degrade this fusion protein, is a critical drug for treating APL.2 In pancreatic ductal adenocarcinoma, RARα and RARβ were shown to be downregulated, which is associated with worse survival outcomes.3 The cytoplasm of hepatocellular cancer cells shows an increased expression of RARγ, which engages with P85α. This interaction results in the activation of protein kinase B (AKT) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), thereby enhancing cell growth and survival.4, 5 Furthermore, RARγ overexpression in the nucleus enhances stem cell pluripotency and self-renewal by regulating the expression of a large network of genes, including those targeted by the important transcription factors SOX2, NANOG and POU5F1 in stemness.6, 7 Research conducted in vitro indicates that inhibiting RARγ could be a promising approach for addressing cancer metastasis and recurrence.8 In addition to cancer, dysregulation of the RAR signalling network plays a critical role in developmental eye defects and cartilage breakdown in patients with osteoarthritis.9, 10 Furthermore, impairment of RAR/RXR signalling also contributes to the development of diabetic cardiomyopathy.11, 12
Given the critical role of RARs in disease occurrence and development, novel drugs targeting specific RAR subtypes are currently under development. SY-1425, a selective RARα agonist, demonstrated efficacy in non-APL acute myeloid leukemia (AML) patients with superenhancers at the RARα gene locus and elevated levels of RARα mRNA.13 Retinoids combined with standard chemotherapy have also been proposed to improve survival rates in non-APL AML patients, particularly in those with specific mutations, such as NPM1 mutations.14 Besides, some patients with myelodysplastic syndromes have shown improvement with retinoid therapy, indicating a potential role for retinoids in promoting differentiation and possibly reversing the malignant phenotype in these conditions.15 In addition to blood system diseases, the activation of RARs is also involved in the treatment of other cancers. Acyclic retinoids were shown to prevent the recurrence of hepatocellular carcinoma (HCC) by targeting MYCN+ cancer stem cells.16 In recurrent HCC, the synthetic retinoid sulpharotene (WYC-209) activated RARα, leading to the downregulation of SOS2, which subsequently affected RAS activation and inhibited tumour formation mediated by tumour repopulating cells (TRCs).17 Furthermore, sulpharotene was demonstrated to inhibit TRC proliferation in intrahepatic cholangiocarcinoma by inducing RARα translocation from the cytoplasm to the nucleus and suppressing the transcription of P-selectin.18 Additionally, sulfarotene activated the caspase-3 pathway, directly inducing apoptosis in TRCs in malignant melanoma.19 Prior to apoptosis, RARγ bound to the Cdc42 promoter in the nucleus and subsequently translocated to the cytoplasm, resulting in the downregulation of Cdc42 expression and a decrease in filamentous actin, thereby inhibiting cytoskeletal tension in TRCs.20 Another synthetic retinoid, ZSH2208, was found to inhibit TRC proliferation in esophageal squamous cell carcinoma by modulating the RARγ‒TNFAIP3 axis.21 In addition to cancer, Palovarotene was shown to prevent heterotopic ossification in patients with fibrodysplasia ossificans progressive by disrupting bone morphogenetic protein signalling pathways.22 CD5789 has received international approval for topical use in treating acne vulgaris, as its high selectivity for RARγ enables it to effectively target skin cells, promoting differentiation and reducing acne lesions without side effects.23, 24
Despite the availability of various synthetic retinoids that target RARs and exhibit anticancer effects, those with higher selectivity and potency remain to be explored. This review emphasises the crystal structures of RARs as they activate, a key factor in developing therapies aimed at RARs. Additionally, we discuss the structural features of ligand-bound (holo) RARs when complexed with different types of RAR modulators.
2 RARS FUNCTION AND STRUCTURE
The initiation of transcriptional control in target genes occurs through RAR/RXR heterodimer interaction with specific retinoic acid response elements (RAREs) positioned within gene promoter regions.25, 26 In the absence of ATRA, this binding recruits co-repressors, along with histone deacetylases (HDACs). HDACs deacetylate histones, increasing their positive charge and promoting chromatin condensation, thereby inhibiting transcription.27 ATRA binding triggers allosteric transitions in RAR that lead to dissociation of transcriptional co-repressors while simultaneously exposing docking sites for co-activatos.28, 29 These co-activators recruit additional factors, including histone acetyltransferases, which acetylate histones and lead to chromatin decondensation.29, 30 This relaxed chromatin enables the transcription machinery, including RNA polymerase II, to access the promoters of target genes, initiating mRNA transcription and facilitating gene expression.28
RARs play a multifunctional role in cells, particularly in cellular development and differentiation.31 Throughout the process of embryonic development, gene expression is regulated by RAR, which is essential for the formation of various organs and tissues. For instance, in neural system development, RAR participates in hindbrain patterning by activating Hox gene expressions, such as Hoxa1, Hoxb1 and Hoxd3.32, 33 In organogenesis, RAR is essential for organ morphogenesis.34 In eye development, RAR controls periocular mesenchymal growth by regulating Pitx2 gene and inhibiting WNT signalling pathways.35 In neural system differentiation, RAR promotes specific neuronal cell type differentiation, particularly in the basal ganglia, where it supports dopaminergic and GABAergic neuronal differentiation.36, 37 In reproductive systems, RAR plays a critical role in spermatogenesis, especially in inducing Stra8 gene expression and initiating meiotic processes.38 In cardiac and hepatic development coordination, RAR indirectly promotes cardiac development by regulating erythropoietin (Epo) gene expression.39, 40
Regarding their two-dimensional structure, RARs, similar to other NRs, display a segmented configuration consisting of six unique regions marked A‒F (Figure 1A). The A/B regions, which represent the N-terminal domain, show considerable variability among the different RAR subtypes and encompass activation function 1 (AF-1). This function engages with co-regulators to maintain the basal transcriptional activity of unliganded RARs. Given that no specific structures are available, the A/B domains are now regarded as a naturally disordered or malleable structures, which may help explain their ability to interact with diverse co-regulators. The C region, referred to as the DNA-binding domain (DBD), represents the most conserved segment of RARs and is essential for the dimerisation of receptors as well as for identifying specific RAREs within target genes. The D region, known for its poor conservation, serves flexibly as a hinge connecting the DBD to the ligand-binding domain (LBD). The E region plays a pivotal role in the RAR signalling process, encompassing several essential components, including the LBD, AF-2, a dimerisation surface, and the binding site for co-regulators. This region is characterised by a notable level of evolutionary conservation, highlighting its fundamental importance across different species. In contrast, the F region, which extends from helix H12 located in the LBD to the C-terminal end of the protein, is recognised for its intrinsic disorder. This disordered region is crucial as it facilitates the binding of co-repressors by maintaining the stability of helix H12. The ability of the F region to enhance the interaction between the receptor and co-repressors underscores its functional significance in the regulatory dynamics of RAR signalling.41 To summarise, domains A/B, D and F exhibit low conservation, whereas domains C and E demonstrate high conservation (Figure 1B).
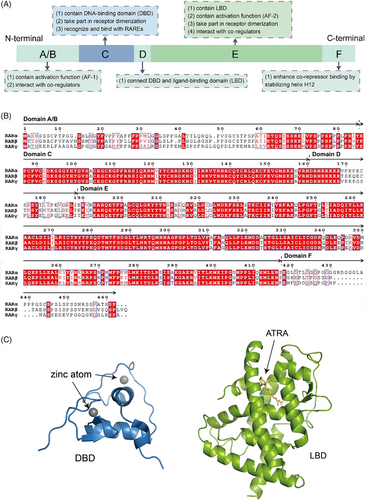
When it comes to three-dimensional conformation, previous studies have well-established the crystal structures of DBD and LBD (Figure 1C). The DBD consists of an N-terminal β-hairpin, two α-helices followed by a single turn of 310 helix and a C-terminal extension, along with two zinc ions. A zinc finger motif is formed by the N-terminal β-hairpin, the first α-helix, and one of the zinc ions, in which the ion stabilises the α-helix so that it binds to the major groove of the DNA half-site. The second α-helix is positioned perpendicularly to stabilise the core of the DBD. Although no specific structure of ligand-free (apo) RAR LBD is available, both apo-RXR LBD and holo-RAR LBD structures were determined. The holo-RAR LBD adopts a three-layer α-helical sandwich fold that is characteristic of the NR superfamily, containing 11 α-helices (H1, H3‒H12) and two β sheets between H5 and H6. H1, H3, H7 and H10‒H11 form two outer layers respectively, with H4‒H6, H8‒H9 packed internally between them in the central layer. In apo-RXR, H12 stretches outward from the LBD core, whereas in holo-RAR bound to agonists, it bends back towards the LBD core.
3 STRUCTURE OF ACTIVE RAR COMPLEX
3.1 RAR forms heterodimer with RXR
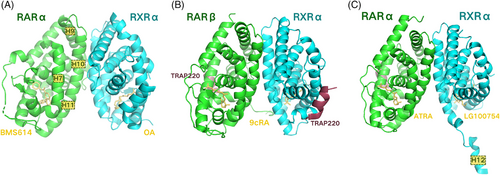
RARs cannot bind directly to DNA on their own. Instead, they form heterodimers with RXRs to modulate transcription. The concrete composition of the heterodimer interface was first described in the RARα‒BMS614/RXRαF318A‒oleic acid heterodimer (PDB ID: 1DKF), involving residues from helices H7 and H9‒H11, and loops L8–9 and L9–10 (Figure 2A). Helices H10 of each protomer within the heterodimer are oriented in a parallel fashion relative to the pseudo twofold axis connecting the two subunits. The interface possesses a hydrophobic core that is encircled by a rim of charged and polar residues. Most of the residues excluded from the solvent and forming the dimer contact region are hydrophilic residues that form various electrostatic and hydrogen bonding interactions, with a smaller fraction consisting of non-polar residues contributing to hydrophobic effects.42 Additionally, the two receptors contribute differently to the contact surface. The crystal structure of the RARβ/RXRα heterodimer (PDB ID: 1XDK) indicated that RARβ and RXRα remained largely unchanged during heterodimerisation, except for some essential sidechain reorientations at the interface (Figure 2B). The two receptors maintain functional independence from each other, preserving their capacities to interact with co-regulators similar to their monomeric counterparts.43 With both receptors bound to agonists, however, there exists a seemingly paradoxical synergy effect, which means the heterodimer containing both subunits in active conformation shows an enhanced binding affinity for the co-activator.44, 45 And the reason behind this lies in the formation of two binding sites that would interact with two LXXLL motifs of a single co-activator molecule.46, 47 Finally, the comparison between the RARα‒ATRA/RXRα‒LG100754 heterodimer (PDB ID: 3A9E) and the one described above (PDB ID: 1XDK) demonstrated that the stabilising interactions, such as hydrophobic contacts, hydrogen bonds and salt bridges, were maintained across various complexes (Figure 2C).43, 48
3.2 Retinoid acid activates heterodimer
In living organisms, endogenous RA attaches to the RAR‒RXR heterodimer to start gene transcription. Without RA, the heterodimer attracts corepressor proteins to inhibit transcription, but it changes to an active form through allosteric modulation. Endogenous RA includes ATRA, 9-cis retinoic acid (9cRA) and 13-cis retinoic acid (13cRA), among which ATRA is the most biologically active RAR agonist, and 13cRA is the least potent.49 The structure of the ATRA-liganded RARγ LBD (PDB ID: 2LBD) was established in 1995, which was also the first RAR crystal structure to be solved (Figure 3A). ATRA takes on a somewhat curved shape that is nestled deep inside a mainly hydrophobic cavity formed by residues located in H1, H3, H5, the β-turn, loop L6‒7, H11, loop L11‒12 and H12 of the RAR LBD. The interaction between H12 and loop L11‒12 causes H3 to bend, allowing W227, F230 and A234 to directly contact ATRA. These additional interactions, together with the inward shift of H6, contribute to the compact structure of the holo-RARγ. The C-terminal H12, which seals the ligand-binding cavity in its final position, acts as a molecular switch that determines whether RAR is in an active or repressive state.50 In 2010, the structure of the RARα‒ATRA/RXRα‒LG100754 LBD heterodimer (PDB ID: 3A9E) was determined, showing that H12 in its ‘closed’ conformation, along with H3 and H4 in RARα‒ATRA, formed a cleft that was recognised by the LXXLL motif of co-activator peptide (Figure 3B).48
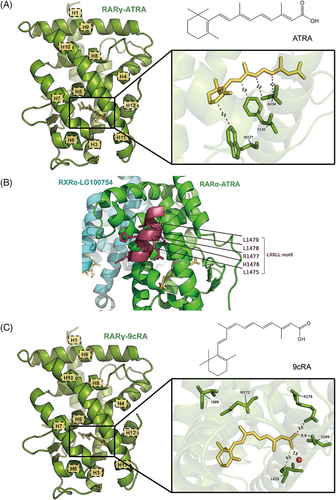
9cRA, a cis-isomer of ATRA characterised by a distinct alkene arrangement at C9, has the capability to activate both RARs and RXRs. The structure of the 9cRA-bound RARγ LBD (PDB ID: 3LBD) is similar to the RARγ‒ATRA described above (Figure 3C). Although 9cRA binds in a more kinked conformation, no residue shifts occur as the β-ionone moiety is able to fit in a small cavity near Ile412. However, the 19-methyl group of 9cRA leads to a displacement of the Met272 (H5) sulphur atom relative to ATRA, which in turn rotates the Ile389 (H10) side chain.51 Met272 is one of the three divergent residues across different subtypes and corresponds to an isoleucine residue in RARα and RARβ. This steric clash is suggested to account for the lower affinity of 9cRA for RARγ.50 The other interactions are similar between RARβ‒9cRA and RARγ‒9cRA, as indicated by the structure of the RARβ‒9cRA/RXRα‒9cRA heterodimer (PDB ID: 1XDK). Moreover, the carboxylate group of 9cRA creates a salt bridge with Arg278 and participates in a hydrogen bond network with Ser289, Leu233 and a water molecule, aiding in the stabilisation of 9cRA's binding in the receptor's binding pocket.43
3.3 Heterodimer binds to DNA at RARE
RAR and RXR possess a highly conserved DBD, enabling both receptors to identify and attach to RAREs found within the regulatory regions of DNA, thereby influencing gene transcription. Typically, RAREs consist of two direct repeats (DR) of 5′-(A/G)G(G/T)TCA-3′-like motif, separated by spacer nucleotide of various length. In most cases, RXR binds to the upstream half-site and RAR binds to the downstream, except DR1 and DR0 complexes, whose polarity are reversed.52-54 The DR5 element, which contains a 5 base pair spacer, is one of the most well-characterised RAREs of the RAR/RXR heterodimer and participates in the activation of gene transcription (PDB ID: 6XWG) (Figure 4A).55 It forms direct and water-mediated interactions with the highly conserved residues in DBDs of both RXR and RAR, including Glu153, Lys156 and Arg161 in RXR, as well as Glu106 and Lys109 in RAR. Besides, RXR Arg172 forms contact with the phosphate group of the spacer's last nucleotide, and RAR interacts with the sugar backbone of the second nucleotide in the downstream half-site. These exact interactions between the DBDs and the DNA sequence's bases or backbone facilitate the specific attachment of the RAR/RXR heterodimer to DR5 sequences. Moreover, a comparison was made between DR5 and DR0 elements, the latter is a common non-canonical element in undifferentiated cells, and its binding to the heterodimer is unable to regulate gene transcription.56, 57 It turned out that DR5 binding, rather than DR0, induced a conformational alteration in the LBD of RXR through the interaction between RAREs and the DBD, which might explain the functional difference between DR5 and DR0 complexes.41
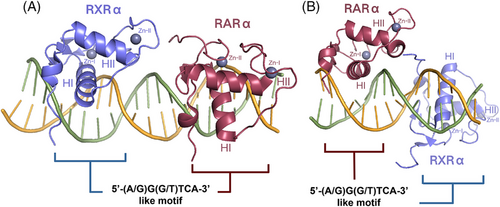
The DR1 element, which has a lower affinity for the heterodimer than the DR5 element, is another type of RARE that repressed gene transcription when bound.52, 58 Similar to the DR5 complex, base-specific interactions and extensive backbone contacts are also formed between the DBDs and DR1 sequences, but those involve different residues (PDB ID: 1DSZ) (Figure 4B). Furthermore, the DR1 DNA helical axis is distorted by the heterodimer binding, mainly at the 5′ half-site, facilitating the interaction of RAR and RXR with their half-sites.59
3.4 Heterodimer recruits co-activators
A primary role of the RAR/RXR heterodimer involves the recruitment of co-activator proteins. Co-activators are proteins that can alter chromatin structure, bring in the basic transcription machinery, and aid in starting gene expression.60 They contain a region known as the nuclear receptor interaction domain (NID) that interacts with the LBDs of the heterodimer.61, 62 The highly conserved LXXLL motifs, also named NR boxes, within the NID are essential for the binding and recruitment of these co-activators.63, 64 When the agonist binds and helix H12 covers the ligand-binding pocket (LBP), the LXXLL motif adopts an α-helical conformation, facilitating the co-activator's attachment to the surface composed of residues from helices H3, H4 and H12 of the LBD.43, 65, 66 A specific crystal structure is described in a RARβ/RXRα heterodimer in complex with the NR box2 of the TRAP220 co-activator in 2005 (PDB ID: 1XDK) (Figure 5A). The peptide folds as a two-turn amphipathic α-helix, interacting with the hydrophobic co-activator binding groove. In both RARβ and RXRα, a conserved lysine located at the C terminus of H3, along with a preserved glutamate in H12, establishes hydrogen bonds with a backbone peptide bond of the LXXLL motif. This interaction results in a charge clamp that determines the precise length of the helical motif that is positioned within the cleft. In RXRα particularly, the binding of the TRAP220 NR box2 induces a concerted orientation of Phe282 in helix H3, Phe442 in helix H11 and Phe455 in helix H12, allowing them to closely interact with helix H12 and TRAP220 peptide. After complex interactions, Phe282 and Phe442 form an ‘aromatic clamp’ that holds helix H12 in the ideal configuration, which stabilises the transcriptionally active RXR conformation.43, 67
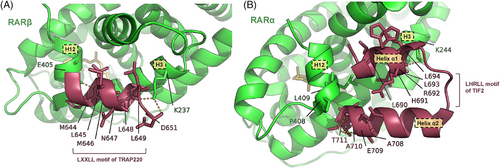
In 2021, TIF2, an intrinsically disordered co-activator, was found to form a helix‒turn‒helix motif during the recruitment (PDB ID: 7APO) (Figure 5B). The helix α1, identified as the LHRLL helix of TIF2 NR box2, engages with the co-activator binding groove of RAR. Additionally, the C-terminal flanking region, which also adopts the structure of an α-helix referred to as helix α2, establishes hydrophobic interactions with residues from both helix α1 and RAR. The above two regions compose a helix‒turn‒helix motif, which allows TIF2 to stably and specifically interact with RAR. However, TIF2 binds to RXR only through helix α1 that is followed by a disordered flanking region.68 The binding mode of RAR and SRC1, a co-activator preferentially acetylating histone H3, is similar to that of RXR and TIF2.43, 68 Therefore, the flanking regions may account for NR specificities of different co-activators.69-71
4 BINDING STRUCTURE OF SPECIFIC RAR MODULATOR
RAR modulators are compounds that selectively bind to and activate or inhibit the activity of one or more RAR subtypes. The binding of these modulators to the RAR triggers structural changes in the receptor, resulting in the involvement of co-activator or co-repressor proteins, which either promotes or suppresses the transcription of target genes.28, 72 Understanding the binding structure of specific RAR modulators is meaningful as it sheds light on the molecular mechanisms that drive the biological effects of these compounds. It also helps elucidate the structure‒activity relationships of RAR modulators, enabling the rational design of new compounds with improved pharmacological properties. Furthermore, the binding structure is also essential for understanding the selectivity and specificity of RAR modulators towards different RAR subtypes, which is the cornerstone for developing more targeted therapies with fewer side effects and better therapeutic efficacy. Herein, we summarise the structural basis of which existing modulators with different selectivity exert agonism or antagonism, and introduce two examples in detail, including a selective RARα antagonist and a selective RARγ agonist.
4.1 Structural characteristics of existing RAR modulators
4.1.1 Structural basis of agonism or antagonism
Despite their different pharmacologic effects, RAR agonists and antagonists are both able to fit into the LBP, thus sharing similarities in their structures.10, 42, 73-75 The overall structure of most synthetic retinoids includes a cyclic core, a carboxyl group and a hydrophobic tail. The core structure of RAR modulators typically resembles that of retinoic acid, which is generally a cyclic or polycyclic aromatic system, often a cyclohexenyl or phenyl ring. At one end of the molecule, there is usually a polar carboxylic acid group. This group forms hydrogen bonds and salt bridges with an arginine residue in helix H5 and a serine residue in β-turn of the LBD, which is essential for the binding of the ligands. The opposite end of the molecule typically features a hydrophobic tail, which varies in length and saturation. This part interacts with the hydrophobic LBP, aiding in the stabilisation of the complex. The exact nature of the hydrophobic tail influences the selectivity and effect of the compound.51, 75-77 Moreover, most RAR modulators possess a conjugated system involving double bonds that extend from the core aromatic ring, which affects the planarity and rigidity of the molecule.74, 78, 79 Stereochemistry which involves the spatial arrangement of atoms, is also an influencing factor.75, 80, 81 In addition to the basic parts listed above, functional groups including hydroxyl, ether, ester or additional carboxylic acid groups, as well as ring substitutions by methyl groups or halogens, make a significant difference to the pharmacokinetic properties of the modulator and are largely responsible for the final function of the ligand.10, 78, 81
The interaction of RAR agonists leads to a sequence of conformational alterations, ultimately resulting in the movement of helix H12, which aids in the secure closure of the LBD and the creation of the co-activator-binding interface.51, 65, 66, 78, 82 RAR antagonists, however, prevent the receptor from adopting an active conformation through steric hindrance, failing to form a functional surface for the co-activators and even favouring the corepressors instead.10, 73 It is worth noting that unlike the single LBD structure adopted by agonist-bound RARs, the LBDs bound to various antagonists might be different from each other but all results in the silence of gene transcription.42
4.1.2 Structural basis of different selectivity
Distinct roles in various biological processes are associated with different subtype of RARs, making the selective targeting of these receptors essential for therapeutic strategies. The structural characteristics of ligands that exhibit selectivity towards different RAR subtypes are designed to exploit subtle differences in the LBDs of these receptors.
The three divergent residues in the LBP of different RAR subtypes are primary targets for the design of ligands with specific selectivity. They are Ile270, Val395, Ser232 in RARα, Ile263, Val 388, Ala225 in RARβ and Met272, Ala397, Ala234 in RARγ (Figure 6A).50, 83 The different conformation of these residues imposes steric effects and prevent the binding of modulators with specific functional groups. For example, the branched side chains of Ile270 in RARα and Ile263 in RARβ occupy more space and have lower tolerance for steric volume associated with ligands compared to the linear side chain of Met272 in RARγ.10 Thus, introducing functional groups at the corresponding position leads to the relative selectivity for RARγ. Some ligands, such as CD564, also generate a steric hindrance with the side chain of Ser232 in RARα, resulting in RARα discrimination.75 On the other hand, Ile263 also contributes to the larger volume of RARβ LBP, with its side chain orientation opening a cavity between helix H5 and H10, which allows the binding of more bulky modulators such as BM641.74, 78
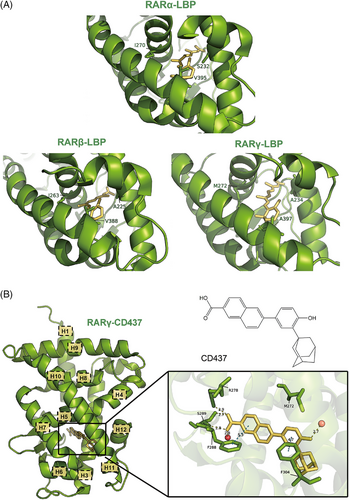
In addition, these residues are able to form contacts with the ligands, accounting for the specific selectivity. The most important one of these is Met272 in RARγ. This methionine possesses a sulphur atom in its side chain that forms hydrogen bonds with hydroxyl or oxime groups in selective ligands.24, 75, 80, 81 Besides, Met272's side chain exhibits flexibility, allowing ligands like SR11254 to adopt dual Z/E tautomeric states. Both tautomers form O‒H‒S hydrogen bonds with Met272, while additional C‒H‒O interactions with Ile275 further stabilise binding.80 However, CD437 is an exception (PDB ID: 6FX0). This ligand pushes Met272 away and causes a shift in its side chain, creating a new small sub-pocket that contains two water molecules. The phenol group of CD437 forms a strong hydrogen bond with these water molecules instead of Met272 itself, thus maintaining its RARγ selectivity (Figure 6B).24
Finally, the subtype-specific residues also have a knock-on effect. BMS948, for instance, exhibits selectivity for RARβ over RARα due to a unique amide linker conformation. The carbonyl group of BMS948 points towards Ala225 in RARβ rather than Ser232 in RARα, which results in a 180° flip of the amide bond compared to its orientation in RARα, thus altering the position of the ligand's rings and affecting its binding affinity.78
4.2 Specific examples of existing RAR modulators
4.2.1 Selective RARα antagonist: BMS614 (PDB ID: IDKF)
BMS614 is a selective RARα antagonist that consists of an ‘agonistic’ core and an ‘antagonistic’ quinolyl group.42 The ‘agonistic’ core is buried in the RARα LBP similarly to ATRA, except for several slightly modified side chain orientations (Figure 7A).50 However, the shorter length of BMS614 compared to ATRA releases the steric hindrance to some extent, thus pulling helix H11 into the LBP by 10.7°. The ‘antagonistic’ quinolyl group, which establishes a hydrogen bond with a water channel originating from the protein surface and extending through a cavity, is oriented in a nearly perpendicular manner relative to the ‘agonistic’ core and protrudes from the LBP of the receptor between helices H3 and H11, creating a steric clash with Ile412 of helix H12. Therefore, rather than capping the entrance of the LBP, helix H12 binds to the groove that is supposed to interact with the co-activator NR boxes, making the co-activator-binding surface unable to form.47, 69, 84 Except for the steric hindrance, the tilting motion of helix H11 and the longer L11‒12 loop of RARα compared with RARγ also help stabilise helix H12 in this antagonist conformation.42
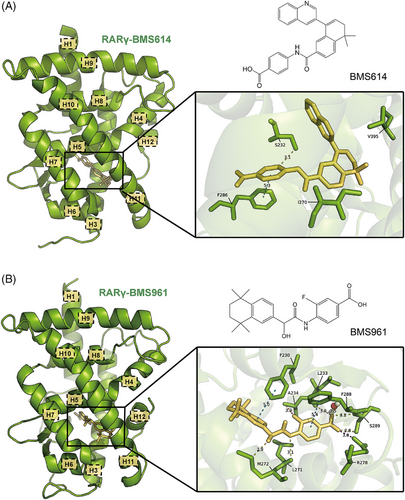
The interactions between BMS614 and the three subtype-specific residues of RARα account for the selectivity of BMS614, although some contacts differ from those of other RARα-selective modulators. For instance, Ser232 OH is hydrogen bonded with the aromatic CH instead of amide NH or carbonyl groups. The absence of this serine in RARβ and RARγ leads to lower affinities. Moreover, in RARγ specifically, Met272, which corresponds to RARα Ile270, causes a steric hindrance, and Ala397 corresponding to RARα Val395, disrupts a stabilising contact. Both of these result in the low affinity between BMS614 and RARγ.42
4.2.2 Selective RARγ agonist: BMS961 (PDB ID: 4LBD)
BMS961 is a selective RARγ agonist, whose binding structure is similar to that of 9cRA (Figure 7B).85-87 At the molecule's terminus, the carboxylate group of benzoic acid engages in interactions similar to those of 9cRA, creating a salt bridge with Arg278 located in helix H5. Additionally, it is involved in a hydrogen-bonding network that includes Ser289, Leu233 and a water molecule, which is essential for its receptor binding. Moreover, BMS961 exhibits a bend similar to 9cRA, with a tilt between its aromatic rings, allowing it to conform to the natural curvature of the RARγ LBP. As the pocket's structure is conserved, BMS961 can fit snugly and induce the same active conformation of helix H12.51
BMS961 also forms subtype-specific interactions within the RARγ LBP. Similar to other RARγ-selective modulators, the hydroxyl group present in BMS961 establishes an essential hydrogen bond with the sulphur atom of Met272. In addition, the fluorine atom shows brief van der Waals interactions with Ala234 located in helix H3, while the amide nitrogen is connected via hydrogen bonding to Leu271, which also plays a role in its selectivity. On the other hand, Ala397 in RARγ allows room for the hydrophobic TTN moiety of BMS961, while the bulkier valines at this position in RARα/β lower the affinity. RARα also has a serine (Ser232) in helix H3, which would sterically clash with BMS961, while Ala234 in RARγ avoids this clash. Therefore, both the interactions and steric effects above lead to BMS961's selectivity towards RARγ.51
5 CONCLUSION AND PERSPECTIVE
Given the indispensable role of RARs in cell differentiation and other biological processes, the development of RAR modulators based on their crystal structure is urgent. In this review, we systematically explore the crystal structures of RARs through various stages of activation and interaction, from their unliganded state to their activated state, wherein they form heterodimers with RXRs and recruit co-regulators. We also delve into how these heterodimers bind to DNA at RAREs, ultimately modulating gene transcription, and how specific RAR modulators influence these interactions. The elucidation of RAR structures via crystallography sheds light on the molecular basis of their activation and the specificity of ligand binding. This understanding is crucial for illustrating how different ligands modulate RAR activity and how activated RARs initiate gene transcription. The binding structures of specific modulators demonstrate how subtle variations in ligand structure can lead to significant changes in receptor function, highlighting the potential for designing subtype-specific drugs.
The field of RAR structural biology presents significant potential for developing novel therapeutic agents. Detailed knowledge of the four RAR activation steps and the ligand‒receptor interaction mechanisms creates opportunities to develop selective RAR modulators targeting specific RAR subtypes or distinct phases of RAR activation. Further studies ought to focus on broadening the structural database of RARs bound to ligands, co-regulators, RXRs, and DNA to enhance our understanding of how RARs function in the onset and progression of various diseases. RARs form large complexes with multiple factors to regulate transcription; however, existing crystal structures focus primarily on a limited number of these factors, indicating that substantial work remains to be done. Consequently, artificial intelligence (AI) holds significant promise in the field of RAR crystal structure research. AlphaFold-like deep learning models, trained on nuclear receptor structures, can predict unknown RAR variants and refine existing structures. Graph neural networks, which analyse ligand‒protein interactions more accurately than traditional docking software, can predict the binding modes of retinoic acid derivatives or synthetic ligands. Additionally, hidden regulatory sites on RARs may be identified using 3D convolutional networks, revealing novel targets for allosteric modulators and enabling the development of drugs with fewer adverse effects. AI can also facilitate drug design and generate specific RAR modulators with optimised properties. Furthermore, more advanced imaging techniques should be developed and adopted to provide higher-resolution images of RAR complexes in states that are challenging to crystallise, thereby offering deeper insights into transient interactions and dynamics.
Ultimately, significant challenges remain in translating detailed structural knowledge into clinical applications. This process entails not only the development of RAR-targeted therapies but also a comprehensive understanding of retinoid signalling, including its functions across a range of diseases and its interactions with other signalling pathways.
AUTHOR CONTRIBUTIONS
Xin Cao conceived the concept and scope of the review. Yining Song, Wenrui Zhao, Xuan Huang, Lai Wei, Jingyi Han, Jiayun Hou and Min Li performed the literature review, organised and prepared the manuscript. Yining Song, Wenrui Zhao, Min Li, Jiayun Hou and Xin Cao revised the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82373719 and 82173662). The authors would like to thank all members of the laboratory for their critical review of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
No ethical approvals were required to produce this review article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




