Towards a one-time cure for Fabry disease: Lentivirus-mediated haematopoietic stem and progenitor cell gene therapy
Fabry disease, also known as Anderson‒Fabry disease, is an X-linked systemic disease first described independently in Germany and England over 125 years ago, in 1898, in patients presenting with small, reddish purple telangiectatic cutaneous papules called angiokeratoma corporis diffusum.1 Five decades later, in 1947, the concept of a lipid storage disease emerged following postmortem studies, and the abnormal lipid was identified as trihexosyl ceramide in 1963.1 Subsequently, the underlying deficiency of the lysosomal enzyme α-galactosidase, which cleaves galactose from the neutral glycosphingolipid, globotriaosylceramide (Gb3) or trihexosyl ceramide, in the normal cellular degradation pathway, was identified.1, 2 The enzyme activity was near-completely deficient in leukocytes in affected males and reduced in female carriers of the disease compared to normal leukocytes.2 In 1978, the α-galactosidase A (GLA) gene, which encodes the enzyme, was found to be located in the Xq22 chromosomal region, and the gene's nucleotide sequence was identified in 1986. Gene mutations in GLA that do not allow enzyme expression were recognised as the fundamental cause of Fabry disease.3
Although the clinical features of this inherited disease, including neurologic pain in the extremities, cutaneous angiokeratomas, hypohidrosis, and corneal opacities, are evident in childhood, the diagnosis of classic Fabry disease is often missed and delayed, with an average age of 29 years at diagnosis.3, 4 The disease worsens in untreated adults due to the progressive accumulation of glycosphingolipids in various cells across multiple organ systems, leading to cardiovascular, cerebrovascular, and renal damage that shortens lifespan. Females exhibit variability in disease severity and may be asymptomatic or experience complications like affected males. Until 2001, no treatment was available to halt disease progression, when enzyme replacement therapy (ERT) was approved for treating Fabry disease in Europe, followed by approval in the United States in 2003.4 Nonetheless, ERT is very expensive, requires biweekly infusions for life, and its efficacy may be reduced if the patient develops an antibody response. Migalastat, a pharmacologic chaperone therapy approved for adults with Fabry disease since 2016, is only applicable for 30%–50% of patients who have GLA mutations that are amenable to increase the activity of the deficient enzyme with the drug; furthermore, the in vitro determinations of amenability do not translate to in vivo drug efficacy.5
Medin et al. began their gene therapy efforts more than two decades ago with the vision of developing a one-time cure for Fabry disease.6, 7 In an accompanying article in this journal, Medin's team described the 5-year end-of-study results from the Canadian Fabry Disease Clinical Research and Therapeutics (FACTs) trial, which was the first gene therapy trial—single-arm, non-randomised phase I clinical trial (NCT02800070) of ex vivo recombinant lentivirus-induced autologous haematopoietic stem and progenitor cell (HSPC)-mediated—to be completed for Fabry disease.8, 9 In this therapy, the virus-induced bone marrow-derived HSPCs with functional GLA differentiate into circulating myeloid cells, which secrete α-galactosidase A that acts by metabolic co-operativity or cross-correction, as depicted in Figure 1.6, 8 The enzyme uptakes into the uncorrected cells in various tissues in a mannose-6-phosphate receptor-dependent manner, allowing it to degrade the accumulated glycosphingolipid substrates within non-haematopoietic cells.6
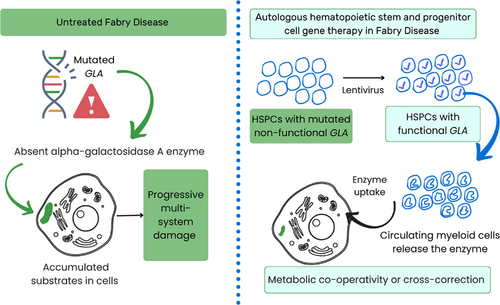
Previously, the preclinical mouse models of this therapy demonstrated even higher levels of α-galactosidase activity with the lentivirus-induced CD34+-enriched HSPCs compared to normal CD34+ HSPCs, alongside a concurrent reduction in substrate accumulation.7 The FACTs trial was initiated in 2016 to evaluate the safety of the lentiviral gene therapy developed by the authors over at least the past 25 years for Fabry disease. Five adult males with four distinct classic Fabry disease mutations confirmed by genotyping, all of whom were receiving ERT, were enrolled. All patients had their bone marrow HSPCs collected and underwent non-myeloablative conditioning with reduced-intensity melphalan. Stronger myeloablation was deemed unnecessary since, for metabolic diseases, even partial improvements in enzyme levels might be enough to discontinue ERT. The CD34+-enriched HSPCs were transduced with a recombinant lentivirus that induced the expression of α-galactosidase A. The patients received the transduced CD34+-enriched HSPCs via infusion as the gene therapy. Circulating α-galactosidase activity began to appear within 6–8 days of the infusion, and this activity remained durable for 5 years in all patients. Leukocyte α-galactosidase-specific activity was comparable to enzyme activity in plasma. The vector copy number, which estimates the average number of integration sites per cell, was analysed in the leukocytes of all treated patients, and this number stabilised throughout the study period. Deacetylated Gb3 (lyso-Gb3) is recognised as a metabolite that is more soluble than plasma Gb3 and correlates with Fabry disease outcome; α-galactosidase A expression decreased the levels of plasma Gb3 in four of five patients and stabilised the renal symptoms in all patients.8 After an initial transient rise in anti-α-galactosidase A antibody titres in plasma in three patients, the reactivity decreased early in the trial, and the antibody titres remained at or near baseline beyond 18 months. All five patients were eligible to discontinue ERT: three of five elected to do so. The therapy was well-tolerated. Only two severe adverse events occurred in the treatment phase,9 with two additional adverse events documented since then.8 The trial was designed as a safety study and was not adequately powered to investigate correlations between gene therapy and specific clinical parameters.8 After haematologic malignancies occurred in patients in a gene therapy trial for another disease, the investigators performed additional experiments to study viral integration sites following gene therapy.10 These studies provided evidence for persistent polyclonal haematopoiesis and no evidence of clonal dominance in any patient.10
Notably, ERT discontinuation in three patients alone saved the Canadian health system $4.8 million; these savings will increase over the 15-year follow-up period. In their carefully planned work, in addition to demonstrating safety features, Medin's group showed the feasibility of a multisite model for designing and delivering gene therapy and conducting the trial in an academic university setting. Their future efforts to include more patients, including at a younger age and females, will undoubtedly be eagerly anticipated by the Fabry disease community worldwide towards the long-standing goal of achieving a one-time cure for this disease.
AUTHOR CONTRIBUTIONS
The author is solely responsible for drafting and writing this article.
CONFLICT OF INTEREST STATEMENT
The author declares she has no conflicts of interest.
FUNDING INFORMATION
This work did not receive any funding from any source.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




