Effects of microRNA on the growth and targeted therapy response on lung cancer
Abstract
Lung cancer represents a significant public health concern worldwide. Lung cancer typically receives a diagnosis at a late stage, leading to a generally unfavourable prognosis. Additionally, traditional treatments frequently fail in cases of metastatic lung cancer. However, targeted therapy has advanced considerably in the management of lung cancer, and overcoming drug resistance has emerged as a significant hurdle in achieving optimal treatment outcomes. As a result, there has been a new trend toward precision therapy for lung cancer based on changes at the molecular and genetic levels. On the other hand, for lung cancer, early diagnosis plays a crucial role in treatment and prognosis. Based on existing knowledge, we strongly believe that it is imperative to promptly identify innovative biomarkers. The emergence of microRNAs (miRNAs) provides new ideas. The expression profiles of miRNAs have been investigated using noninvasive blood samples to explore the regulatory mechanisms played by miRNAs during the progression and targeted therapy resistance of lung cancer. Due to the complexity of miRNA profiles, they may play the role of tumour suppressors or oncogenes. However, specific regulatory mechanisms are still a huge topic to be explored. In this Review, we summarize the latest research that has shed light on the potential regulatory mechanisms of miRNAs in driving lung cancer progression, their value for clinical application as biomarkers and their role in targeted therapy resistance.
1 INTRODUCTION
Globally, cancer is responsible for the majority of fatalities. Among all types of cancer, lung cancer stands out as the most prevalent and widespread. And the incidence of lung cancer has continued to rise worldwide.1 Lung cancer, a devastating disease, holds the unfortunate title of being the primary cause of cancer-related fatalities worldwide, affecting both sexes. In China specifically, it stands as the most prevalent and deadliest form of malignant tumours.2, 3 With the aid of light microscopy, pathologists classify lung cancer into two primary categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).4 One of these two, NSCLC represents a significant category of lung malignancies, encompassing various subtypes. It can be further classified into three primary histological types: squamous cell carcinoma, adenocarcinoma and large cell carcinoma. NSCLC constitutes approximately 85% of lung cancers, while SCLC makes up about 15%.5
The last decade has witnessed transformative breakthroughs in the areas of screening, diagnosing and treating lung cancer. Low-dose CT (LDCT) is a routine method for lung cancer screening.6 Although this method is low cost and simple to perform, the high false positive rate has been one of the main reasons for its limited development. Therefore, there is an urgent need for a more targeted testing technique to enhance lung cancer screening. Current lung cancer diagnosis relies on different types of imaging tests supplemented by pathologic evaluation of biopsies, however, these methods still cannot identify the advancement of early-stage lung cancer.7 With the advancement of research related to precision medicine, lung cancer diagnosis is also moving towards the molecular and genetic level. Meanwhile, precision therapy for lung cancer has significantly improved the quality and effectively extended patient survival time. Currently, the array of therapeutic strategies available for lung cancer includes a comprehensive range of interventions. These strategies consist of surgical removal of the lesion, systemic chemotherapy, localized radiotherapy, as well as the latest emergence of immunotherapy and targeted therapy. In recent years, systemic therapies have advanced rapidly, and the development of molecularly targeted therapy agents, immune checkpoint inhibitors and anti-angiogenic drugs has especially transformed this field and significantly improved patient prognosis.8 Among them, although targeted therapy has made significant advancements in lung cancer treatment, drug resistance has become the biggest obstacle affecting the effectiveness of treatment.
MicroRNAs (miRNAs) are closely related to cancer prognosis, pathogenesis, diagnosis and treatment and their role in cancer is gradually being explored. miRNAs are short, single-stranded RNA molecules consisting of 18–22 nucleotides that derive from chromosomal encoding. They can post-transcriptionally regulate multiple genes through complementary pairing.9 Non-coding RNAs, like transcription factors, regulate gene expression. They participate in various functions, including cell development, proliferation, differentiation, apoptosis, survival, tumorigenesis and metastasis. Since their discovery, researchers have continued to explore their biological functions and involvement in pathological processes, including cancer.10 In cancer, miRNAs can act as tumour suppressors or tumour inducers. Studies have shown that miRNA-based therapies are effective in cancer treatment by inhibiting an oncomiR or by inducing a tumour suppressor.11 The literature frequently reports that the expression of miRNA in various cancers has undergone significant changes. miRNA is a significant class of biomarkers. It can be utilized for cancer screening, diagnosis and prognosis.
The persistent challenges in lung cancer management underscore the promising role of miRNA in the diagnosis, treatment and prognosis of this condition. We summarize the utilization of miRNAs as biomarkers in lung cancer as well as the regulatory mechanisms of miRNAs in the targeted therapy. We anticipate offering enhanced options for lung cancer treatment and expanding the use and effectiveness of targeted therapies.
2 OVERALL OF miRNA
2.1 Biogenesis and mode of action of miRNAs
miRNAs play a key role in gene regulation.12 Recently, miRNAs have gained significant attention for their crucial involvement in tumorigenesis. They regulate key physiological processes, including cell cycle progression, apoptosis, angiogenesis and metabolic pathways. The typical biogenesis of miRNAs is a complex pathway that includes both nuclear and cytoplasmic steps. miRNAs originate from extensive precursor types of RNAs, known as primary miRNAs (pri-miRNAs). These pri-miRNAs undergo a critical processing step within the nucleus, where they are converted into precursor microRNAs (pre-miRNAs). This transformation is significantly enabled by the enzyme RNase III. This enzyme operates as part of a specialized molecular assembly known as the Drosha complex. In this context, it works synergistically with a double-stranded RNA-binding protein, referred to as Pasha. Pre-miRNAs are subsequently translocated into the cytoplasmic compartment through the action of a nuclear transport receptor, which is named Exportin 5. Once in the cytoplasm, these pre-miRNAs undergo a series of enzymatic modifications and processing events that ultimately result in the formation of fully mature miRNAs. Following this maturation process, a singular mature miRNA strand is seamlessly integrated into the silencing complex induced by RNA, known as RISC. miRNAs are crucial for the complex regulation of gene expression. Primarily through mechanisms of post-transcriptional control that affect messenger RNA (mRNA) stability and translation.13 The primary method involves binding to complementary sequences within the 3′ untranslated region (3′-UTR) of the target mRNA. This results in gene silencing by either degrading the mRNA or blocking its translation14, 15 (Figure 1).
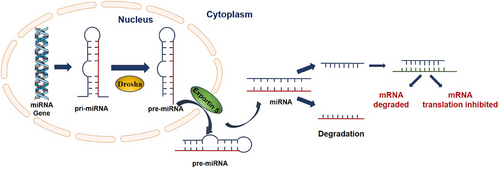
2.2 miRNAs in lung cancer
miRNAs serve as potent gene regulators. A single miRNA can influence entire cellular processes by interacting with numerous target genes. This makes miRNAs a highly promising therapeutic tool. However, the multi-targeting nature of miRNAs is also one of the major reasons limiting the clinical application of miRNAs. Since they have so many effects on cells, it is difficult to avoid off-target effects.16 Almost all types of tumours show altered miRNA profiles compared to normal cells or tissues.17 miRNA dysregulation occurs frequently in cancers. Depending on the situation, they may act as tumour suppressors or oncogenes. Numerous research data suggest that miRNAs have tumour suppressor, carcinogenic and diagnostic effects on lung cancer18, 19 (Figure 2).
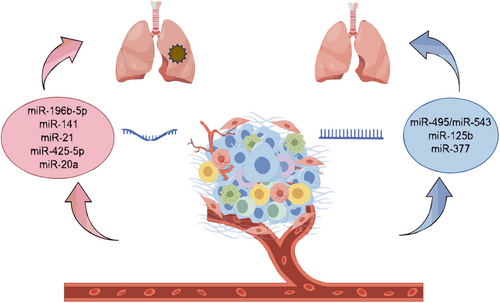
2.2.1 Tumour oncogenes
Tumour growth and invasion are regulated by multiple genes. Various studies have shown that miRNAs can regulate relevant signalling pathways and thus play a role in tumour progression. For example, Liang et al.20 found that the QKI-5 which exerted tumour-suppressive functions in NSCLC could negatively regulate miR-196b-5p. The elevated expression of miR-196b-5p in NSCLC enhances cellular migration and proliferation. MiR-196b-5p could directly target the tumour suppressors GATA-binding factor 6 and Tetraspanin 12. This process enhanced cell migration, increased proliferative capacity and stimulated tumour growth both in vitro and in vivo. On his part, Wang et al.21 demonstrated that miR-141 could target binding to the 3′ UTR region of the growth arrest-specific homologous chromosome gene (GAX) and thus downregulated the expression of GAX. According to Bai et al.,22 compared to paraneoplastic tissues and normal lung cells, miR-21 expression increased in lung cancer tissues and cells. MiR-21 was expressed in the lung cancer tissues and cells in the paraneoplastic tissues and normal lung cells. Down-regulation of miR-21 inhibited tumour cells, whereas up-regulation of miR-21 promoted tumour cells. MiR-21 could regulate lung cancer cells by modulating the Wnt/β-catenin and K-ras pathway. Zhou et al.23 confirmed that miR-425-5p was highly expressed in both lung cancer cell lines and tissues. This suggested that it played an important role in lung cancer tumorigenesis. Up-regulation of miR-425-5p levels improved in vitro survival and colony-forming ability of lung cancer cells. This result suggested that it may be a novel lung cancer oncogene. Phosphatase and tensin homolog (PTEN) were identified as a direct gene target by predictive binding assays. Its exogenous expression inhibited the pro-carcinogenic effects of miR-425-5p. MiR-425-5p activated the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) axis through down-regulation of PTEN. Similarly, Gong et al.24 showed that miR-20a reduced PTEN expression levels while increasing programmed cell death-1 levels, thereby fostering the proliferation of NSCLC cells.
These studies above offer fresh insights for selecting treatment strategies for lung cancer. We can find oncogenic miRNAs and inhibit tumour progression by suppressing their expression or altering the miRNA profile (Table 1).
2.2.2 Tumour suppressors
On the other hand, miRNAs can act as tumour suppressors. According to Tan et al.,25 the miR-495/miR-543 acted as a suppressed factor and was regulated by smad4. In turn, it inhibited the p21-activated kinase 3 (PAK3)-Jun N-terminal kinase (JNK)-Jun pathway, thus exerting a suppressed effect. Similarly, Xu et al.25 reported that Cochlioquinone B derivative (CoB1) induced the expression of miR-125b. It achieved this through the activation of the transforming growth factor beta-activated kinase 1 (TAK1)/ mitogen-activated protein kinase kinase 4 (MKK4)/c-JNK/small mothers against decapentaplegic (Smad) axis and then downregulated the expression of the transcription factor forkhead box protein 3 (Foxp3), thereby enhancing autophagy in lung cancer. MiR-125b triggered autophagy in lung cancer through its sponge-like interaction with Foxp3. This conclusion was further substantiated by evidence showing that silencing miR-125b elevates Foxp3 levels and suppresses autophagy. Hashemi et al.26 also reported that miR-377 acted as a tumour suppressor. It hindered NSCLC progression by negatively regulating the expression of epidermal growth factor receptor (EGFR), p21-activated kinase 2 (PAK2) and mitogen-activated protein kinase 1 (MAPK1). They used bioinformatics analyses to examine the interaction of miR-377 with EGFR, MAPK1 and PAK2. Their findings were validated by luciferase reporter assays, affirming that miR-377 targeted these genes directly. Furthermore, they found that miR-377 expression significantly diminished in NSCLC tissues and lung cancer cells compared to normal tissues. They also observed a notable increase in mRNA expressions of EGFR, MAPK1 and PAK2 in NSCLC tissues. After transfecting miR-377 in Calu-6 and A549 cells, they observed a considerable decrease in mRNA expressions of EGFR, MAPK1 and PAK2. The transfection of miR-377 had shown that miRNA could reduce lung cancer cell proliferation and promote apoptosis.
The reviewed literature showed that in addition to acting as tumour promoters, miRNAs serve as crucial factors in the advancement of lung cancer, functioning effectively as tumour suppressors. Determination of miRNA status may help stratify patients for treatment regimens associated with personalized therapies (Table 2).
2.3 miRNAs as biomarkers
With the continuous updating and maturation of testing technology, liquid biopsy has gradually become an important diagnostic tool for advanced lung cancer. Since miRNAs are involved in almost all of the essential signalling pathways, including the expression of many important tumour-associated genes such as oncogenes and tumour suppressors. Therefore, miRNAs have become one of the most promising biomarkers. The source of circulating miRNAs linked to cancer remains uncertain. They could be released by circulating tumour cells, primary site tumour cells, or cells that have metastasized.27 Several studies have shown that radiation therapy, chemotherapy and targeted therapies affect circulating miRNAs. miRNAs may serve as biomarkers to assess such sensitivity of different therapies, which play an important role in patient management decisions, reduction of unnecessary therapeutic toxicity and efficacy evaluation.28, 29
In recent years, numerous studies have focused on the feasibility of miRNAs as tumour biomarkers. A case-control study30 demonstrated that miR-21 expression levels were significantly upregulated in NSCLC, suggesting that miR-21 may play an oncogenic role in NSCLC and be a poor prognostic factor. Thereby demonstrating that circulating miR-21 expression signature is a potential biomarker for early detection of NSCLC. In a different study, researchers developed a molecular beacon (MB) based assay to detect NSCLC miRNA.31 The researchers designed an MB to target the upregulated miR-21-5p. The data showed that the developed MB method has the potential to detect miR-21-5p in peripheral blood mononuclear cells from NSCLC clinical samples. Cho et al.32 revealed that the expression of miR-145 significantly decreases in the plasma of patients with NSCLC. Meanwhile, in vitro experiments indicated that increased levels of miR-145 reduced the proliferation, migration and invasion of NSCLC cells. They also demonstrated that miR-145 mimics notably slowed the growth of tumours in vivo. This result was consistent with this in plasma. In another study, Grenda et al.33 suggested that miR-126 may be a predictor of the efficacy of first-line immunotherapy or chemoimmunotherapy in NSCLC patients, as found in a two-stage study of a validation group (20 patients) and a study group (35 patients) of patients with advanced NSCLC.
All the aforementioned studies indicate that miRNAs serve as diagnostic biomarkers while also playing a crucial role in therapy and prognosis. miRNAs possess several advantages as biomarkers. They can be extracted from liquid biopsies, which are less invasive than lung biopsies, offering better accessibility, safety and reproducibility. Moreover, miRNAs exhibit a longer half-life compared to other circulating biomarkers. Additionally, they can be integrated with chest CT scans to detect tumours at earlier stages during diagnosis or in later treatment phases.
3 TARGETED THERAPY IN LUNG CANCER
The emergence of precision medicine has illuminated the treatment landscape for lung cancer patients. Targeted therapies informed by genetics and epigenetics have broadened the therapeutic choices for those battling advanced lung cancer. However, due to the incomplete understanding of the aetiology and pathogenesis of SCLC, along with its considerable heterogeneity, targeted drug options for SCLC remain limited. However, targeted therapy is still one of the directions to be explored in the future treatment of SCLC. Current targeted therapy for lung cancer is mainly for NSCLC. Tumour driver genes have been identified in NSCLC patients one by one, including EGFR, mesenchymal lymphoma kinase, receptor tyrosine kinase ROS proto-oncogene 1, Kirsten rat sarcoma viral oncogene homolog, receptor tyrosine kinase, V-RAF mouse sarcoma virus oncogene homolog B1, mesenchymal-epithelial transition factor (MET) and vascular endothelial growth factor.34-36 Despite significant advances in available therapies for NSCLC, a major hurdle of acquired resistance remains.37 Resistance to precision-targeted therapy can occur in two forms: preexisting and adaptive. Clinically, these are identified as primary and acquired drug resistance. First, tumour cells may possess genetic alterations that offer resistance to specific targeted inhibitors. Second, the signalling pathways within tumour cells can demonstrate redundancy, which allows for bypass mechanisms in oncogenic signalling, leading to incomplete pathway suppression. Third, histological or phenotypic transitions of lung adenocarcinoma to small cell carcinoma significantly contribute to acquired resistance in lung cancer. This involves early adaptive drug escape through cellular adaptive “histological” reprogramming, enhancing plasticity in response to therapy.38-40
In response to this therapeutic dilemma, on the one hand, we can further develop new targeted drugs. For example, three generations of Food and Drug Administration-approved EGFR tyrosine kinase inhibitors (EGFR-TKIs) have been developed for EGFR mutations, and a fourth generation of drugs will be launched soon. On the other hand, new therapies offer more possibilities for targeted therapy. These include proteolysis-targeting chimaera therapy, which utilizes the intracellular ubiquitin-proteasome system to induce degradation of targeted proteins,41 and miRNA therapy, which focuses on the large family of miRNAs. miRNA therapy, in turn, is one of the great treasure troves that is currently in high demand due to its wide variety.
4 IMPACT OF miRNAs ON TARGETED THERAPY FOR LUNG CANCER
Lung cancer treatments have reached a plateau. This stagnation arises from our limited grasp of lung cancer's pathogenesis and the variability in tumour gene expression profiles. Therefore, genomic medicine is an emerging field in this direction, which will bridge the research on the pathogenesis of lung cancer.42 Combined application schemas of targeted therapy and miRNA manipulators are especially being developed for the improvement of antitumor therapies. As researchers continue their investigation into miRNAs, they have discovered that these molecules play a crucial role in regulating cancer cell metabolism and can influence resistance or sensitivity to chemotherapy, radiation and targeted therapies43 (Figure 3).
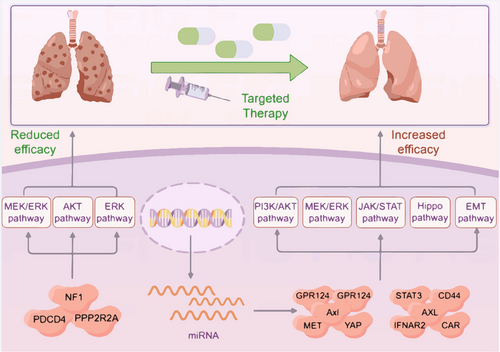
There are various treatments for NSCLC, including chemotherapy, traditional Chinese medicine, molecular targeted therapy and surgery. Therapeutic progress for subgroups of NSCLC can largely be attributed to the development of new drugs that specifically target molecular abnormalities.44
4.1 miRNAs related to targeted therapy resistance
With advancements in gene sequencing technology, researchers are now examining gene-driven mutations. This progress led to the emergence of targeted drug therapy, offering renewed hope for patients with particular gene mutations. Targeted therapy shows great promise and has yielded positive outcomes in treating advanced lung cancer. However, the issue of drug resistance remains a significant challenge in lung cancer treatment. Therefore, if we can thoroughly understand the new mechanisms of resistance to targeted therapy, it could greatly enhance the clinical application of these therapeutic strategies.
Huang et al.45 study provided data that the inhibition of mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signalling overcomes Osimertinib resistance in vitro and in vivo. Leveraging the TCGA database, they developed a model of cancer-associated fibroblasts (CAFs) by co-culturing NSCLC cells from patients with various susceptibilities to Osimertinib alongside human lung fibroblasts. Mechanistically, the research revealed that the MEK inhibitor trametinib attenuated the tumorigenic traits of NSCLC cells by diminishing miR-21 expression and influencing the regulation of programmed cell death 4 (PDCD4), which subsequently lowers CAF generation. This study confirmed that miR-21 inhibits PDCD4 expression and thus promotes NSCLC progression.
Gu et al.46 proposed that elevated levels of plasma miR-136-5p are linked to poor clinical outcomes with anlotinib. They also discovered that miR-136-5p is significantly present in NSCLC cells resistant to anlotinib. Their experiments confirmed that miR-136-5p enhanced proliferation and contributed to anlotinib resistance in A549 cells by targeting the regulatory subunit of protein phosphatase 2A (PP2A) regulatory subunit (PPP2R2A), resulting in AKT pathway activation. This finding suggested that miR-136-5p could be a potential biomarker to evaluate response to anlotinib in NSCLC patients.
Chen et al.47 found elevated levels of miR-641 in TKI-resistant NSCLC cells and human NSCLC samples, compared to TKI-sensitive counterparts. They explored the mechanism by which miR-641 fosters resistance to targeted therapy through both in vitro and in vivo studies. Their research indicated that stimulating ERK signalling by focusing on neurofibromatosis 1 and enhancing miR-641 expression significantly contributed to the emergence of erlotinib resistance in NSCLC cells. Conversely, inhibiting miR-641 diminished the acquired resistance of NSCLC cells to erlotinib.
The literature review indicates that miRNAs significantly correlate with resistance to targeted therapy in lung cancer. More studies are now concentrating on the regulatory roles that miRNAs play in this context. This suggests that we can clinically inhibit miRNA expression that is positively associated with resistance to targeted therapy in order to delay drug resistance (Table 3).
4.2 miRNAs enhance targeted therapy sensitivity
Due to the large variety of miRNAs, in addition to those positively associated with resistance to targeted therapy in the above studies, there are numerous miRNAs that can enhance the sensitivity to targeted therapies.
For example, Gao et al.48 demonstrated through cellular assays that miR-138-5p levels declined in gefitinib-resistant cells compared to sensitive ones. Their findings indicated that miR-138-5p, which is plentiful in gefitinib-sensitive PC9 cells, directly suppressed the expression of G protein-coupled receptor 124 (GPR124), thereby enhancing gefitinib sensitivity. Conversely, upon acquiring gefitinib resistance, miR-138-5p expression diminished, resulting in elevated GPR124 expression. Clinical data further corroborate these findings.
Liu et al.49 demonstrated that the direct targeting of cyclic RNA hsa_circ_0005576 to miR-512-5p was clarified by dual luciferase assay. They found that miR-512-5p acted as a tumour suppressor that targeted the insulin-like growth factor 1 receptor (IGFR1). Overexpression of transfected miR-512-5p increased the sensitivity of H1975OR cells to osimertinib by down-regulating IGFR1 expression, thereby inhibiting their colony-forming ability. This study provided a novel insight into the mechanisms underlying osimertinib resistance of lung adenocarcinoma.
Similarly, Xiong et al.50 reported that dysregulated miR-34a could affect the related downstream signalling pathways PI3K/AKT, MEK/ERK and the Janus kinase/signal transducer and activator of transcription (STAT) via Axl (Anexelekto, a member of the receptor tyrosine kinase sub-family), thereby modifying tumorigenesis and gefitinib resistance in NSCLC both in vitro and in vivo. First, they identified an inverse relationship between miR-34a and Axl using cellular assays. After that, they developed a nude mouse model and injected a lentivirus expressing miR-34a through the tail vein in tumour-bearing mice. This method led to a significant decrease in tumour size when compared to the control group. Additionally, the expression levels of proteins involved in the downstream pathway significantly decreased. These results suggested that increasing miR-34a expression could enhance the responsiveness of gefitinib-resistant NSCLC cells to gefitinib therapy.
In another study, Jiao et al.51 conducted RNA-seq and bioinformatics analysis to uncover the differential expression of miR-1/133a and miR-206/133b in gefitinib-resistant and sensitive cell lines. They utilized lentiviral vectors to promote overexpression of Hepatocyte Growth Factor (HGF) and created stable gefitinib-resistant lung cancer cell lines. Following this, in vitro assays demonstrated that the miR-1/133a and miR-206/133b clusters lowered MET protein levels and enhanced the suppression of AKT and ERK activities. This methodology effectively tackled HGF-driven gefitinib resistance in NSCLC with EGFR-sensitive mutations.
Chen et al.52 elucidated that miR-7 served as a critical suppressor in gefitinib resistance. Their research revealed that miR-7 targets yes-associated protein yes-associated protein (YAP), an important component of the Hippo pathway. This pathway frequently showed overexpression in human epithelial cancers and played a vital role in tumour progression. MiR-7 alleviated gefitinib resistance by binding to YAP, thereby altering the in vitro resistance profile of H1975 cells. Furthermore, they noted increased miR-7 levels in serum exosomes from healthy subjects compared to those with lung cancer. Additionally, high miR-7 expression correlated with robust treatment responses and improved survival in lung cancer patients undergoing gefitinib therapy. These results suggested that miR-7 could serve as a promising biomarker and therapeutic target to tackle drug resistance in lung cancer. They identified miR-7 as a potential regulator of the Hippo pathway, which is often overexpressed in human epithelial tumours and contributes significantly to cancer development. MiR-7 countered gefitinib resistance by interacting with YAP and modified the in vitro resistance characteristics of H1975 cells. In addition, they observed substantially higher levels of miR-7 in serum exosomes from healthy individuals compared to lung cancer patients. High miR-7 expression was linked to enhanced treatment responses and longer survival for lung cancer patients undergoing gefitinib therapy. This lays a foundation for exploring miR-7 as a potential biomarker and therapeutic avenue for addressing drug-resistance mutations in lung cancer.
Yu et al.53 showed that miR-17-5p directly targeted STAT3 by luciferase assay. In this study, researchers cultured gefitinib resistance PC9 cells in vitro to mimic gefitinib resistance in lung cancer patients and verified that miR-17-5p reduced STAT3 protein levels and phosphorylation, inhibited cell proliferation and promoted apoptosis, which ameliorated gefitinib resistance in NSCLC. These preliminary preclinical findings supported this axis as a potential new target for preventing or reducing gefitinib resistance.
Wu et al.54 revealed that lung cancer patients with higher miR-204 levels or elevated serum concentrations showed longer median survival when treated with EGFR-TKIs, compared to those with lower miR-204 levels. Their research confirmed that miR-204 alleviated osimertinib resistance by down-regulating CD44 signalling and inhibiting cancer stemness alongside the epithelial-to-mesenchymal transition (EMT) phenotype. The interaction between miR-204 and CD44 proved crucial in overcoming osimertinib resistance in lung cancer, thereby establishing a foundation for the development of therapeutics aimed at reversing osimertinib resistance.
A study by Du et al.55 showed that the overexpression of miR-625-3p inhibited transforming growth factor-β1-induced EMT and enhanced gefitinib sensitivity by directly targeting AXL in lung cancer cells. Researchers identified that the miR-625-3p/AXL interaction in HCC827GR cells, without the T790M mutation, served as a crucial mechanism for drug resistance. These discoveries provided new therapeutic approaches focused on improving drug sensitivity and addressing resistance to EGFR-TKIs.
Zheng et al.56 demonstrated that levels of prostate cancer-associated transcript 6 (PCAT6) increase in gefitinib-resistant NSCLC. Reducing PCAT6 expression decreased gefitinib resistance in NSCLC. Additionally, PCAT6 could interact with miR-326 in gefitinib-resistant NSCLC cells. Interferon-alpha receptor 2 (IFNAR2) was identified as a target of miR-326, which alleviated gefitinib resistance by lowering IFNAR2 expression. This discovery may offer a new treatment strategy for addressing gefitinib resistance in NSCLC patients.
Wang et al.57 provided that the overexpression of the constitutive androstane receptor (CAR) in NSCLC increased the resistance of NSCLC cells to targeted therapies. In contrast, miR-4271-infected NSCLC cells showed heightened sensitivity to targeted agents such as Gefitinib. Furthermore, miR-4271 improved the sensitivity of NSCLC to Gefitinib in vivo by down-regulating CAR expression. These findings indicated that miR-4271 may play a key role in developing more effective treatment strategies for advanced NSCLC.
The literature review revealed that miRNAs play a role in regulating various tumour-associated pathways, and these pathways significantly influence the sensitivity to targeted therapy in lung cancer.58 Since overexpression of these miRNAs can enhance the sensitivity of targeted therapies for lung cancer, we can reasonably envision the feasibility of applying “miRNA therapy” in the clinic. It could be a strategy to overcome resistance to primary and acquired targeted therapy in NSCLC (Table 4).
| miRNA | Molecular targets | Possible mechanism | Targeted drugs | Reference |
|---|---|---|---|---|
| miR-138-5p | GPR124 | Unknown | Gefitinib | [48] |
| miR-512-5p | IGFR1 | Unknown | Osimertinib | [49] |
| miR-34a | Axl | PI3K/AKT MEK/ERK JAK/STAT | Gefitinib | [50] |
| miR-1/133a miR-206/133b | MET |
AKT ERK |
Gefitinib | [51] |
| miR-7 | YAP | Hippo | Gefitinib | [52] |
| miR-17-5p | STAT3 | Unknown | Gefitinib | [53] |
| miR-204 | CD44 | EMT | Osimertinib | [54] |
| miR-625-3p | AXL | EMT | Gefitinib | [55] |
| miR-326 | IFNAR2 | Unknown | Gefitinib | [56] |
| miR-4271 | CAR | Unknown | Gefitinib | [57] |
5 CONCLUSION AND EXPECTS
The emergence of targeted therapy has brought new hope to patients with advanced lung cancer. Significant progress has occurred in the domain of targeted lung cancer therapies. The introduction of these therapies has markedly enhanced the clinical outcomes for lung cancer patients with driver gene mutations. However, the challenge of drug resistance frequently accompanies the use of targeted treatments. Therefore, overcoming the challenge of drug resistance in targeted therapy is crucial in the lung cancer treatment landscape. Responding to this resistance, researchers are developing new targeted agents while simultaneously uncovering rare targets. On the other hand, targeted therapy in combination with other therapies can achieve the desired efficacy. The gathering of molecular insights via advanced technology platforms, including next-generation sequencing and other omics tools, introduces novel avenues for researching drug resistance in targeted lung cancer therapies.
miRNAs offer new ideas in terms of improved efficacy of targeted therapies. Meanwhile, miRNAs have clinical applications as biomarkers in the prediction, prognosis and efficacy evaluation of lung cancer. The emergence of emerging technologies such as high-throughput sequencing has enabled comprehensive molecular characterisation of various non-coding RNA expression profiles in multiple cancers.
Although future therapeutic applications of miRNAs are undoubtedly attractive, there are still significant practical difficulties to overcome. These include determining the appropriate route of administration, controlling their stability in vivo, targeting specific tissues and cell types and achieving the desired intracellular effects. As a result, only a few miRNA-based drugs have entered clinical trials to date. miRNAs are a great treasure trove for the modern treatment of lung cancer. Understanding how miRNAs promote metastasis will provide evidence for developing new targeted treatments.
ACKNOWLEDGEMENTS
This work was funded by the Shanghai Municipal Health Commission, grant number: 202140370, the Natural Science Foundation of Shanghai, grant number: 21ZR1463700, Shanghai Clinical Research Center of Traditional Chinese Medicine Oncology, Science and Technology Commission of Shanghai Municipality, grant number: 21MC1930500 and the National Natural Science Foundation of China, grant number: 82374533.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
No ethical approval was required as it did not involve the collection or analysis of data involving human or animal subjects. And all authors conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




