Pharmaceutical company payments to Japanese breast cancer practice guideline authors
Abstract
Background
The creation of breast cancer practice guidelines requires proper management of financial relationships with drug companies, as they can introduce conflicts of interest (COIs) among guideline authors. However, little is known about the specific landscape and fraction of financial interactions between the authors of the Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer, edition 2022 (JBCS2022) and drug companies.
Methods
Using payment data publicly disclosed by major drug companies in Japan, this study analysed the personal payments made to the authors of JBCS2022 between 2016 and 2020. We performed descriptive analyses on the payment data.
Results
Of the 149 JBCS2022 authors, 115 (77.2%) received at least one personal payment totaling $3 828 455 from drug companies between 2016 and 2020. The average and median payment amounts per author were $25 772 (standard deviation: $58 197) and $2761 (interquartile range: $322‒$15 828), respectively. The total annual payments per JBCS2022 authors between 2016 and 2019 increased from $588 054 in 2016 to $967 802 in 2019. The JBCS2022 chairperson and vice-chairperson received $246 936 (fourth highest) and $216 744 (fifth highest) over the 5 years. More than 60% of personal payments to the JBCS2022 authors were not declared by the authors as they were below the declaration threshold set by the Japanese Breast Cancer Society. However, nine authors undeclared personal payments summing $594 615 even though these payments were higher than the thresholds.
Conclusion
This study demonstrated that the majority of the breast cancer guideline authors received personal payments from drug companies in Japan. Furthermore, the majority of payments were not declared because of the less transparent COI policy.
1 INTRODUCTION
Clinical practice guidelines (CPGs) play a crucial role in establishing standardised diagnostic and treatment protocols established to treat patients by endorsing evidence-based medicine.1, 2 However, compiling evidence suggests that the financial conflicts of interest (COIs) between drug companies and the CPG authors potentially compromise the credibility of the guidelines.3-13 These concerns jeopardise the trust in drug companies and draw attention.1, 2, 5, 14-18 Given the profound impact of CPGs on patients, clinicians and various stakeholders, and their perception as guiding principles for physicians,19 rigorous COI management strategies, encompassing minimisation of COIs among authors and guideline-producing societies, full disclosure of financial COIs and the appointment of COI-free CPG chairs, are indispensable. Such measures are imperative for cultivating reliable CPGs.2, 15, 20, 21
Among several types of cancer, breast cancer is the most common cancer, with more than 2.3 million patients having newly diagnosed breast cancer and 685 000 deaths in 2020.22 In Japan, the number of patients with breast cancer was estimated at 97 142 in 2019, the most commonly diagnosed cancer among females.23 There have been remarkable advancements in breast cancer treatments as well as increased prevalence of early diagnosis.24-26 Recent research in the UK showed that the 5-year mortality rate for breast cancer significantly decreased from 14.4% during 1993−1999 to 4.9% during 2010−2015.27 Similarly, the 5-year survival rate increased from 84.6% in 1993−1996 to 92.4% in 2009−2011 in Japan.23, 28, 29
Furthermore, the field of breast cancer treatment has undergone a significant paradigm shift in the last decade with the introduction of breakthrough drugs such as human epidermal growth factor receptor 2 (HER2) inhibitors, poly(ADP-ribose) polymerase inhibitors and immunotherapies. Alongside the introduction of these novel drugs, the global market for breast cancer drugs has been growing substantially, reaching around $26.4 billion in 2022. However, limited head-to-head evidence regarding the safety and effectiveness of these novel drugs30-33 has led to fierce competition among manufacturers and increased marketing activities for physicians.34-36 A previous study reported that 78.2% of oncology CPG authors received personal payments for lecturing, consulting and drafting fees from drug companies in Japan in 2016.37 Considering these fierce marketing activities by the drug companies, we hypothesise that the CPG authors for breast cancer would have financial interactions with drug companies in Japan.
2 METHODS
2.1 Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer
We performed a cross-sectional analysis using the personal payment data made by the Japan Pharmaceutical Manufacturers Association (JPMA) member companies to authors of the Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer, edition 2022 (JBCS2022), for the lecturing, counselling and writing proposals between 2016 and 2022. JPMA is the largest pharmaceutical trade organisation in Japan, and all drug companies belonging to JPMA are required to disclose payments to health care professionals and health care organisations on each company website under the Transparency Guideline for the Relation Between Corporate Activities and Medical Institutions.38
All authors of the JBCS2022 were included. The JBCS, established in 1964, is the largest and most influential association for physicians specialised in breast cancer treatment. The JBCS first published its Clinical Practice Guideline in 2002 and continues to update to provide current recommendations to support the decision-making process in systemic, surgical, radiation treatment and diagnostic methods for early and metastatic breast cancer in Japan. There were two sections of JBCS2022: treatment section and epidemiological and diagnostic section. We considered all JBCS2022 authors in both sections. The study involved a total of 149 unique authors who contributed to the development of the JBCS2022.
2.2 Data collection and payment source
The full names and affiliations of all JBCS2022 authors are publicly available from the official website of JBCS.39 Payments for giving lectures, consulting services and manuscript writing made to JBCS2022 authors between 2016 and 2020 were extracted from an online transparency database containing all payments disclosed by drug companies belonging to JPMA,40 as in previous studies.3-5, 8, 9 All drug companies affiliated with the JPMA are demanded to report payments concerning lecturing, writing and consulting with the individual names of the payment recipients. Other payment categories, such as food and beverage fees, travel and accommodations fees, and royalties and licensing fees, are disclosed in aggregated values and are not able to analyse the individual payment for each author. The personal payments for giving lectures, consulting services and manuscript writing are generally provided directly to the guideline authors from drug companies, and in larger values than those for other types of payments. Additionally, the drug companies update and remove previous years' payment data. Since 2016, the Medical Governance Research Institute has collected payments information voluntarily from drug companies disclosing payment data and published a searchable payment database containing these payment data.40 As of October 2023, the personal payments made in 2020 were the most recent available and analysable information in Japan. Thus, considering the nature of personal payments, this study analyses personal payments for lecture, consultancy and writing fees from 2016 to 2020.
2.3 Data analysis
First, we performed descriptive analyses of the personal payments, including average with standard deviation (SD) and median with interquartile range (IQR), for individual JBCS2022 authors and drug companies. Furthermore, payments were categorised into three main types: lecturing, consultancy and writing, according to JPMA disclosure guidance.38
Second, the study also investigated the proportion of authors who received payments exceeding specific thresholds, $10 000, $50 000, $100 000 and $250 000, as previously described.3, 41, 42
Third, we reviewed the JBCS COI policy for the JBCS2022 authors and the JBCS2022 authors self-declared their financial COIs with drug companies between 2019 and 2021 following the JBCS COI policy. We evaluated their COI statements regarding lecturing, consulting and writing payments with the payment data that we collected from the database between 2019 and 2020. According to the JBCS2022 FCOI disclosure policy, all authors have to self-declare receipt of payments for lecturing, consulting and writing fees exceeding annual payments of ¥500 000 ($4682) per company. Thus, we considered 2019−2020 payments exceeding ¥500 000 to an author from a company not listed in the COI statements as undeclared payments.
Finally, the study identified the top drug companies that made the largest payments to the JBCS2022 authors. Then, we searched the Pharmaceuticals and Medical Devices Agency website,43 the organisation responsible for reviewing and approving drugs and medical devices in Japan, to identify drugs approved for breast cancer in Japan between 2010 and 2022 and manufactured by the top-paying companies.
These payments were converted from Japanese yen to US dollars using the average monthly exchange rate of ¥106.8 per $1 in 2020. The data extraction and analyses were conducted using Microsoft Excel (version 16.0, Microsoft Corp.) and Stata version 17.0 (StataCorp).
2.4 Ethical clearance
Since this research was a retrospective analysis of publicly available information from non-human participants, institutional board review and approval were required for this study in Japan in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labor and Welfare.
3 RESULTS
3.1 Summary of payments to the JBCS2022 authors
We identified 149 JBCS2022 authors. Of them, 115 (77.2%) received at least one payment from drug companies totaling $3 828 455 between 2016 and 2020 (Table 1). The average and median payment values were $25 772 (SD: $58 197) and $2761 (IQR: $322‒$15 828) per author over the 5 years, respectively. The large gap between mean and median payments per author indicates that only the small portion of authors received large values of payments from drug companies. The maximum payments per author were $317 291, which were received by the Epidemiology and Prevention Subcommittee chairperson. The JBCS2022 chairperson and vice-chairperson received $246 936 (fourth highest) and $216 744 (fifth highest) over the 5 years.
| Variables | 2016 | 2017 | 2018 | 2019 | 2020 | Total amounts |
|---|---|---|---|---|---|---|
| Total amount of payments ($) | 588 054 | 682 425 | 808 863 | 967 802 | 781 312 | 3 828 455 |
| Mean payments per author (standard deviation) ($) | 3947 (9769) | 4580 (11 038) | 5429 (12 809) | 6495 (14 705) | 5244 (12 127) | 25 772 (58 197) |
| Median payments per author (interquartile range) ($) | 261 (0‒2816) | 322 (0‒3441) | 313 (0–3164) | 521 (0‒4289) | 319 (0‒3164) | 2761 (322‒15 828) |
| Maximum ($) | 55 500 | 71 945 | 73 745 | 84 839 | 79 726 | 317 291 |
| Authors with payments (N = 149), n (%) | ||||||
| Any payments | 75 (50.3) | 82 (55.0) | 01 (53.7) | 89 (59.7) | 78 (52.4) | 115 (77.2) |
| >$10 000 | 16 (10.7) | 21 (14.5) | 22 (14.8) | 23 (15.4) | 23 (15.4) | 48 (32.2) |
| >$50 000 | 3 (2.0) | 2 (1.3) | 4 (2.7) | 4 (2.7) | 2 (1.3) | 21 (14.1) |
| >$100 000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (8.1) |
| >$250 000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.0) |
- Note: Japanese yen (¥) were converted to US dollars ($) using the 2020 average monthly exchange rate of ¥106.8 per $1.
For the payment category, payments for lecturing were the largest, $3.0 million (79.6% of overall payments), and the most frequent was the 3710 (79.4%) payment categories (Table 2). Of all the authors, 74.8% of the authors received one or more payments for lectures from drug companies, while 38.8% of the authors received payments for consulting and drafting fees over 5 years.
| Variables | Payment amounts (%) ($) | Number of payments, n ($) | Number of authors receiving payments, n (%) |
|---|---|---|---|
| Total amounts of payments | 3 828 455 (100) | 4674 (100) | 149 (100) |
| Payment categories | |||
| Lecturing payments | 3 045 841 (79.6) | 3710 (79.4) | 110 (74.8) |
| Consulting payments | 530 479 (13.9) | 639 (13.7) | 57 (38.8) |
| Writing payments | 252 135 (6.6) | 325 (7.0) | 57 (38.8) |
| Top 10 companies making the largest payment amounts | |||
| Chugai Pharmaceutical | 795 542 (20.8) | 1061 (22.7) | 75 (50.3) |
| AstraZeneca | 490 994 (12.8) | 598 (12.8) | 60 (40.3) |
| Pfizer Japan | 490 164 (12.8) | 496 (10.6) | 54 (36.2) |
| Eisai | 390 538 (10.2) | 537 (11.5) | 64 (43.0) |
| Eli Lilly Japan | 330 878 (8.6) | 383 (8.2) | 41 (27.5) |
| Kyowa Kirin | 228 203 (6.0) | 274 (5.9) | 44 (29.5) |
| Novartis Pharma | 184 023 (4.8) | 278 (5.9) | 51 (34.2) |
| Daiichi Sankyo | 164 828 (4.3) | 223 (4.8) | 41 (27.5) |
| Bayer Yakuhin | 157 793 (4.1) | 148 (3.2) | 20 (13.4) |
| Taiho Pharmaceutical | 127 510 (3.3) | 164 (3.5) | 43 (28.9) |
3.2 Payment trends between 2016 and 2020
Table 1 represents the yearly trends of personal payments to JBCS2022 authors from 2016 to 2020. The total annual payments per JBCS2022 authors between 2016 and 2019 increased from $588 054 in 2016 to $967 802 in 2019 (Table 1). The median annual payment values ranged from $261 in 2016 to $521 in 2019. A slight decline in the total payment to $781 312 (19.3% decrease from the previous year) was observed in 2020. More than 50% of the JBCS2022 authors received payments from drug companies each year from 2016 to 2020. However, the proportion of authors receiving more than $10 000 ranged from 16 authors (10.7%) in 2016 to 23 authors (15.4%) in 2020.
3.3 Undeclared and under-declared financial conflicts of interest
Our analysis revealed that 31 (20.8%) authors disclosed financial COIs with companies for at least COI category. Of 31 authors who disclosed their COIs, 25 (80.6%) declared financial COIs for receiving lecturing and consulting payments.
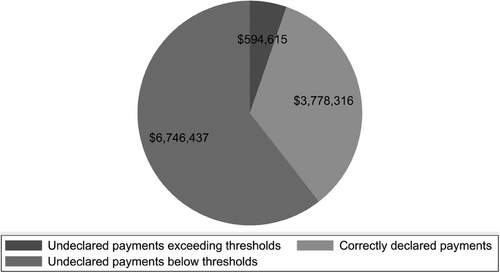
Of a total of $11 119 367 payments for lecturing, consulting and writing made to the authors between 2019 and 2020, $4 372 931 (39.3%) payments exceeded the declaration threshold set by the JSBC (Figure 1), while the remaining 60.7% ($6.7 million) were less than the declaration thresholds. Of the payments exceeding the thresholds, 86.4% ($3.8 million) were correctly declared by 16 authors. However, nine authors received undeclared payments summing $594 615, with median payments of $6109 per author.
3.4 Payments by type of guideline committee
Table 3 represents the payments to the authors by the types of guideline committees. The prevalence of authors' self-declared FCOIs was more than 50% for only the General Guidelines Committee and the Pharmacotherapy Subcommittee. However, subgroup analysis by type of guideline committee showed that 88.9% of the member authors of the General Guideline Committee received at least one payment, with the highest median payment of $114 153 (IQR: $2944–$216 744). All authors of the Pharmacotherapy Subcommittee and Surgical Therapy Subcommittee received personal payments, with median payments of $38 075 (IQR: $16 443–$79 571) and $13 453 (IQR: $2944–$27 230), respectively. Authors of the Radiotherapy Subcommittee and Systematic Review Committee received the lowest payment amounts with medians of $900 (IQR: $0–$1360) and $477 (IQR: $0–$1584), respectively. There were committee chairpersons receiving more than $100 000 in 5-year payments from four JBCS2022 subcommittees.
| Committee type | Number of authors self-declared conflicts of interest, n (%) | Number of authors receiving any payments, n (%) | Mean payments (standard deviation) ($) | Median payments (interquartile range) ($) | Payments to committee chairperson ($) | Maximum payments ($) |
|---|---|---|---|---|---|---|
| General Guideline Committee (N = 9) | 5 (55.6) | 8 (88.9) | 121 301 (117 622) | 114 153 (2944 – 216 744) | 246 936 | 317 291 |
| Pharmacotherapy Subcommittee (N = 20) | 12 (60.0) | 20 (100) | 58 946 (58 470) | 38 075 (16 443−79 571) | 114 153 | 209 032 |
| Surgical Therapy Subcommittee (N = 10) | 1 (10.0) | 10 (100) | 26 475 (38 016) | 13 453 (2944−27 230) | 55 226 | 123 996 |
| Radiotherapy Subcommittee (N = 8) | 1 (12.5) | 5 (62.5) | 1133 (1501) | 900 (0−1360) | No payment | 4540 |
| Diagnostic and Screening Subcommittee (N = 15) | 3 (20.0) | 13 (86.7) | 17 894 (29 119) | 6173 (1365−22 560) | 2944 | 93 402 |
| Pathology Subcommittee (N = 8) | 2 (25.0) | 5 (62.5) | 4062 (6482) | 1338 (0−5655) | 2851 | 18 513 |
| Epidemiology and Prevention Subcommittee (N = 9) | 2 (22.2) | 9 (100) | 73 019 (130 795) | 4162 (2897−25 775) | 317 291 | 317 291 |
| Guidelines for Patients Subcommittee (N = 6) | 3 (50.0) | 6 (100) | 41 978 (58 970) | 7605 (3568−97 212) | 135 564 | 135 564 |
| Guideline Review Committee (N = 9) | 6 (33.3) | 8 (88.9) | 53 687 (90 787) | 20 783 (1255−40 962) | 96 693 | 281 718 |
| Advisors and External Expert (N = 3) | 1 (33.3) | 2 (66.7) | 42 449 (69 730) | 4422 (0−122 926) | Not applicable | 122 926 |
| Collaborators (N = 2) | Not declared | 1 (50.0) | Not calculated | Not calculated | Not applicable | 322 |
| Systematic Review Committee (N = 58) | Not declared | 34 (59.7) | 1601 (3285) | 477 (0−1584) | Not applicable | 15 828 |
3.5 Payments by companies
Among the 45 drug companies making one or more payments to the JBCS2022 authors, the top five and 10 companies occupied 65.3% and 87.8%, respectively, of the total payments (Table 2). Chugai Pharmaceutical Co., Ltd., made the largest amounts of personal payments, accounting for $795 542 (20.8% of all overall payments) over the 5 years in monetary value, followed by AstraZeneca ($490 994, 12.8%), Pfizer Japan Inc. ($490 164, 12.8%), Eisai Ltd. ($390 538, 10.2%) and Eli Lilly Japan K.K. ($330 878, 8.6%). Furthermore, Chugai Pharmaceutical made payments to the highest number of authors (75 authors, 50.3%), followed by Eisai Ltd. (64 authors, 43.0%) and AstraZeneca (60 authors, 40.3%).
Table 4 shows the drugs of the top five companies that were granted new approval and/or additional indications for breast cancer between 2010 and 2022. Chugai Pharmaceutical Co., Ltd. had five breast cancer granted new and/or additional indications between 2010 and 2022, including trastuzumab (Herceptin), bevacizumab (Avastin), pertuzumab (Perjeta), trastuzumab emtansine (Kadcyla) and atezolizumab (Tecentriq). AstraZeneca manufactured fulvestrant (Faslodex) and goserelin acetate (Zoladex), both for the treatment of hormone receptor-positive, HER2-negative advanced or metastatic breast cancer (https://ascopost.com/News/58264). Pfizer Japan Inc., and Eli Lilly Japan K.K., manufacture CDK4/6 inhibitors, palbociclib (Ibrance approved in 2017) and abemaciclib (Vezenio approved in 2018), respectively.
| Company name | Generic drug name (brand name) | Approval indications and year |
|---|---|---|
| Chugai Pharmaceutical | Atezolizumab (Tecentriq) | For the treatment of PD-L1-positive, hormone receptor-negative, and HER2-negative inoperable or recurrent breast cancer (approved in September 2019) |
| Trastuzumab emtansine (Kadcyla) |
For the postoperative adjuvant treatment for HER2-positive breast cancer (approved in August 2020) For the treatment of unresectable or recurrent HER2-positive breast cancer (approved in September 2013) |
|
| Pertuzumab (Perjeta) |
For the treatment of HER2-positive breast cancer (approved October 2018) For the treatment of unresectable or recurrent HER2-positive breast cancer (approved in June 2013) |
|
| Trastuzumab (Herceptin) | For the treatment of breast cancer with HER2 overexpression (approved in June 2013) | |
| Bevacizumab (Avastin) | For the treatment of unresectable or recurrent breast cancer (approved in September 2011) | |
| AstraZeneca | Olaparib (Lynparza) | For the postoperative adjuvant treatment of BRCA mutation-positive and HER2-negative breast cancer with a high risk of recurrence (approved in August 2022) |
| Fulvestrant (Faslodex) |
For the treatment of breast cancer (approved in September 2017) For the treatment of postmenopausal breast cancer (approved in September 2011) |
|
| Goserelin acetate (Zoladex) | For the treatment of premenopausal breast cancer (approved in March 2014) | |
| Pfizer Japan | Biosimilar trastuzumab | For the treatment of breast cancer overexpressing HER2 (approved in September 2018) |
| Palbociclib (Ibrance) Novartis |
For the treatment of HR-positive and HER2-negative inoperable or recurrent breast cancer (approved in January 2020) For the treatment of unresectable or recurrent breast cancer (approved in September 2017) |
|
| Eisai | Eribulin mesylate (Halaven) | For the treatment of inoperable or recurrent breast cancer (approved April 2011) |
| Eli Lilly Japan | Abemaciclib (Verzenio) |
For the postoperative adjuvant treatment of HR-positive and HER2-negative breast cancer with a high risk of recurrence (approved in December 2021) For the treatment of HR-positive and HER2-negative inoperable or recurrent breast cancer (approved in September 2018) |
- Abbreviations: BRCA, breast cancer gene; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PD-L1, programmed cell death ligand 1.
4 DISCUSSION
This analysis of payment information publicly reported by major drug companies found that the JBCS2022 authors received over $3.8 million from these companies for the 5-year period from 2016 to 2020. More than three-quarters of the JBCS2022 authors had financial interactions with drug companies, particularly those manufacturing novel breast cancer drugs in Japan. Furthermore, substantial values of personal payments were compensated to the JBCS2022 chairpersons and leading authors. While most authors received personal payments below the declaration thresholds of the Japanese Society of Breast Cancer, nine authors had undeclared COIs with drug companies, and more than 60% of payments were not disclosed by the authors in the JBCS2022 due to these thresholds. These study findings highlighted several deviations from current international COI standards in the JBCS2022.
The high prevalence (77.2%) of financial ties to drug companies for non-research purposes among the authors in this study aligns with many previous studies in Japan, albeit with higher percentages.3-9, 13, 41, 42, 44-48 This could indicate a vulnerability to financial COI among the influential breast cancer experts in Japan. For example, the author previously reported that the proportions of authors accepting payments from drug companies were 66.0% in obstetrics and gynecology,13 88% in nephrology,5 88.2% in gastroenterology,3, 45 88.6% in urology,46 90.6% in dermatology,47 91.3%–100% in rheumatology,4, 41 87.0%–91.9% in infectious diseases,6, 44 94.4% in cardiology,48 94.6% in hematology,9 95.6% in a diabetes CPG,42 96.3% in otolaryngology7 and 100% in a CPG for hepatitis C treatment.8
Meanwhile, previous studies in other high-income countries, such as the United States, the UK and Canada, reported lower fraction of COIs for CPG authors than did those among the breast cancer CPG authors in this study.11, 49-55 Desai et al. reported that 45.6% (224 out of 491 authors) of National Comprehensive Cancer Network (NCCN) CPG authors were financially ties to health care companies, including both pharmaceutical and medical device companies, in the United States in 2020.56 Of 47 authors of the NCCN breast cancer CPGs, 19 (40.4%) authors, had financial COIs with health care companies.56 However, this is a result of ongoing efforts by many medical professional societies in these countries to properly manage financial COIs among CPG authors. In 2016, Mitchell et al. reported that 86% of NCCN CPG authors received payments from health care industry.57
The finding that the majority of JBCS2022 authors received personal payments from drug companies over the study period represents a clear deviation from international COI standards. The National Academy of Medicine and the Guidelines International Network, a group of guideline-producing organisations from 47 countries, strongly recommend that the majority of authors should not have any financial COIs.1, 2 Additionally, Lenzer et al. stated that multiple CPG authors with financial COIs was one of the eight indicators that should raise substantial skepticism among CPG readers.15 Nevertheless, we previously reported that the majority of CPG authors across specialties in Japan received personal payments from drug companies.3-10, 41, 45-47, 58, 59 Moreover, previous research has demonstrated the correlation between COIs among guideline developers and recommendations favouring drug companies.60 For example, the Surviving Sepsis Campaign reportedly received funding from drug companies, raising concerns about the potential influence of financial interests on guideline recommendations.14, 61, 62 Thus, the financial relationships between the drug industry and professional medical societies, which have played essential roles in creating CPGs and educating physicians in Japan, could endanger the integrity and trust of the public, patients and physicians in clinical recommendations and professional activities of professional medical societies.63-65
Additionally, the significant disparity between the median and average payments per author suggested that a small fraction of JBCS2022 authors received disproportionately large payments. Notably, the JBCS2022 chairperson and leaders of its subcommittees received considerably higher personal payments than other authors. This practice of assigning chairpersons with substantial COIs deviates from international standards.1, 2, 15 Given the influential role of CPG chairpersons in guideline development and recommendation formulation, it is imperative to appoint experts free from any COIs to these positions, as is the practice in many international medical societies.16, 17, 66
One of the fundamental principles in managing the financial COIs of health care professionals, including CPG authors, is ensuring complete transparency in the financial relationships between health care companies and CPG authors.2, 5, 67-69 However, this study revealed that the JBCS required JBCS2022 authors to declare their financial COIs only if they exceeded certain monetary thresholds (more than $4682 per year per company for lecturing and writing payments). These thresholds resulted in a significant under-declaration of COIs among the CPG authors. A literature review by Ngo-Metzger et al. reported that, as of 2012, half of the COI policies mandated that CPG authors declare all financial COIs, irrespective of monetary value.17 Following these findings, the US Preventive Services Task Force revised their COI policies in 2015, shifting from requiring declarations only for COIs exceeding $10 000 per year to mandating the disclosure of all financial COIs, regardless of amount.17 The JBCS2022's numerous deviations from international COI management standards highlight the immediate need for more transparency and rigorous COI management in JBCS guidelines.
Furthermore, the substantial financial relationships between the JBCS2022 authors and drug companies might cast doubt on the credibility of some JBCS2022 recommendations for breast cancer. Chugai Pharmaceutical contributed $795 542 to more than half of the JBCS2022 authors. For instance, the JBCS2022 strongly recommended the use of atezolizumab (Tecentriq from Chugai Pharmaceutical) with nab-paclitaxel (Abraxane from Taiho Pharmaceutical) for advanced or metastatic PD-L1-positive triple-negative breast cancer (TNBC) patients,39 based on the IMpassion130 trial, which demonstrated an improvement in overall survival in a subgroup analysis of PD-L1-positive patients.70, 71 However, the IMpassion131 trial, a confirmatory phase 3 randomised controlled trial evaluating the efficacy of atezolizumab with paclitaxel compared to paclitaxel alone in PD-L1-positive TNBC patients, found no significant difference in either progression-free survival or overall survival.72 Following these negative results from the IMpassion131 trial, the approval of atezolizumab and nab-paclitaxel for metastatic TNBC patients with PD-L1-positive tumours was voluntarily withdrawn by the manufacturer in the United States in 2021.73 Similarly, atezolizumab was removed from the French early access program in 2020, which allowed patients to access drugs before full approval.74 Moreover, despite all eight clinical trials75-81 reviewed in the JBCS2022 failing to show significant benefits in overall survival, the JBCS2022 recommended bevacizumab (Avastin from Chugai Pharmaceutical) as a first-line treatment for HER2-negative metastatic or recurrent breast cancer.39 Contrary to the JBCS2022 recommendation, the US Food and Drug Administration (FDA) withdrew approval for bevacizumab for metastatic HER2-negative breast cancer treatment in 2011 due to its high risk of severe adverse events and low efficacy.82 Notably, the JBCS2022 did not mention these withdrawals by the FDA. Although the withdrawals of these drugs for breast cancer treatment in the United States have sparked controversy within the academic community worldwide, and the judgments by the FDA may not always be appropriate in other countries with differing patient backgrounds, the substantial financial ties between JBCS2022 authors and the manufacturer call into question the objectivity of these JBCS2022 recommendations including the strong recommendation for the use of atezolizumab. As the developer of the publicly accessible payment database themselves emphasised,83 legally binding policies and consensual governance must be achieved to formulate harmonised physician, patients and industrial relationship, such as creation of legally binding and government-run payment database and utilisation of this payment database by medical professional societies and CPG authors to verify their COI declaration and disclosure. In fact, this approach has already been adopted by several medical professional societies in the United States, including the American Society of Clinical Oncology, one of the most influential oncology societies.5, 84
4.1 Limitations
This study has several limitations. First, the generalizability of our findings to other specialties, disease areas or geographic regions may be restricted by the focus of the CPGs on a single disease. Second, payment records were retrieved from the secondary database maintained by Medical Governance Research Institute. While this database covers almost all personal payment data collected from pharmaceutical companies that are members of the JPMA between 2016 and 2020,40 it is important to note that there are no legal regulations in Japan that require the accurate disclosure of such payments to physicians.85, 86 Consequently, this study cannot completely eliminate the potential for inaccuracies or misreporting in the payment information disclosed. In addition, because payment data are voluntarily self-reported by companies associated with the JPMA, there may be unmeasured financial interactions between JBCS2022 authors and drug companies not affiliated with the JPMA. However, all drug companies manufacturing novel breast cancer drugs authorised by the Pharmaceuticals and Medical Devices Agency between 2010 and 2022 were members of the JPMA and had disclosed their payment data. Therefore, the possibility of substantial financial ties with undisclosed companies is considered low within the context of this study.
5 CONCLUSIONS
In conclusion, we observed that there were the extensive financial interactions between authors of the Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer and drug companies. The JBCS2022 chairpersons received much larger payments than those to other authors from drug companies producing breast cancer drugs, some of which were not recommended in other countries. Furthermore, due to the less transparent COI management strategies, the majority of financial COIs with the drug companies were under-declared by the authors. These findings call for immediate policy reforms to promote transparency, integrity and credibility of breast cancer practice guidelines in Japan. Such improvements in COI management are essential to protect the objectivity of CPG recommendations and to maintain the confidence of both the health care providers managing and treating breast cancer in accordance with JBCS2022 and the patients receiving the treatments.
AUTHOR CONTRIBUTIONS
Conceptualisation, methodology, resources, software, formal analysis, investigation, writing—original draft, writing—review and editing, visualisation and study administration: Anju Murayama. Conceptualisation, methodology, resource, software, formal analysis and writing—review and editing: Kenichi Higuchi. Conceptualisation, writing—original draft and writing—review and editing: Keerthana R. Byreddy. Conceptualisation, methodology, resources, investigation and writing—review and editing: Kugo Hinari. Conceptualisation, methodology, resource, formal analysis, investigation, writing—original draft and writing—review and editing: Yuki Senoo.
ACKNOWLEDGEMENTS
The authors appreciate Ms. Megumi Aizawa, MSE, for her support of the study including data collection and constructive feedback on our study. During the preparation of this work, the authors used ChatGPT version 4.0 to check and correct grammatical and spelling errors. After using this tool, the authors carefully reviewed and edited the content as needed and take full responsibility for the content of the publication.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
As this study was a retrospective analysis of publicly available data and met the definition of non-human subjects research, no institutional board review or approval was required according to the Japanese Ministry of Health, Labor and Welfare's Ethical Guidelines for Medical and Health Research Involving Human Subjects.
DECLARATION OF GENERATIVE AI IN SCIENTIFIC WRITING
During the preparation of this work, the authors used ChatGPT version 4.0 to check and correct grammatical and spelling errors. After using this tool, the authors carefully reviewed and edited the content as needed and take full responsibility for the content of the publication.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this study are available from the Yen For Docs database run by the Medical Governance Research Institute (https://yenfordocs.jp/) and each pharmaceutical company belonging to the Japan Pharmaceutical Manufacturers Association. Due to the privacy protection of CPG authors, the data generated and analysed in the study are available from the corresponding author upon reasonable request.




