The relationship between folic acid and nonalcoholic fatty liver disease
Bing'er Xu and Xinyu Yang contributed equally.
Abstract
In recent years, the incidence of non-alcoholic fatty liver disease (NAFLD) has been increasing, which has become an explosive interest because of the growing impact on world health. NAFLD is the hepatic manifestation of systemic metabolic syndrome (MS), and the umbrella term that comprises a continuum of liver conditions, from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), has a variable course but can lead to cirrhosis and hepatocellular carcinoma (HCC). currently, there is no pharmacological agent that is therapeutically approved for the treatment of this disease. Folic acid (FA) was one of a water-soluble B vitamins, entirely absorbed from the diet. Numbers of clinical studies have confirmed that patients with NAFLD and insulin resistance are often accompanied by abnormal FA. We investigated the potential effects of FA on NAFLD through the metabolic pathways, DNA synthesis and methylation, oxidative stress in liver and intestinal flora. In addition, FA has an effect on HCC or other cancer. Therefore, FA might be a safe and economical potential treatment method for NAFLD.
1 INTRODUCTION
In recent years, the incidence of nonalcoholic fatty liver disease (NAFLD) has been increasing. The global prevalence of NAFLD is estimated to be between 10%–35%, making it a rapidly growing concern in global health.1 NAFLD is the hepatic manifestation of the systemic metabolic syndrome (MS), including a continuum of liver conditions, ranging from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). While the course of NAFLD is variable, it can lead to cirrhosis and hepatocellular carcinoma (HCC).1 The early pathological manifestations of NAFLD primarily involve hepatic fat accumulation, followed by hepatocyte injury and fibrosis. Current research has identified a range of factors that contribute to the NAFLD, including insulin resistance (IR), toxic lipids, oxidative stress, intestinal endotoxins, adipokines and genetic abnormalities. NAFLD is the result of multiple factors working in concert. Furthermore, NAFLD is not only associated with adverse liver outcomes but also strongly linked to an increased risk of cardiovascular disease and malignancies.2
Many drugs are being considered as potential treatments for NAFLD/NASH, such as pioglitazone, metformin, ursodeoxycholic acid, omega-3 fatty acids, statins, liraglutide and pentoxifylline. Additionally, novel targets for the drug development, such as thyroid hormone receptor β (THR-β) agonists, Pan-PPAR agonists, Farnesoid X Receptor (FXR) agonists, Stearoyl-CoA desaturase-1 (SCD1) inhibitors and sodium-glucose cotransporter 2 (SGLT2) inhibitors, have shown some treatment effects on NAFLD NASH, and fibrosis.3 However, the safety, cost-effectiveness and clinical benefits of these drugs still require evaluation. Up to date, no drugs have been approved specifically for the treatment of NAFLD. Therefore, dietary and lifestyle modifications remain the cornerstones of the NAFLD therapy. Given their low cost and established safety profiles, natural products are increasingly being investigated as potential treatment options. Such as vitamins A, D, C, E, and some substances like Spirulina, garlic, epigallocatechin-3-gallate (EGCG), berberine and others.4 Vitamin B group, including folic acid (FA, also called vitamin B9) have also been established as playing a crucial role in the development and progression of NAFLD.
Numbers of clinical studies have consistently demonstrated that patients with NAFLD and IR often exhibit abnormal FA levels.5-11 Notably, supplementation with FA has been shown to improve NAFLD and IR in numerous studies.7, 12 This suggests a potential link between FA and the development and progression of NAFLD. A recent prospective cohort study in the United States found that higher intakes of FA, vitamin B6, and vitamin B12, and their serum concentrations, were inversely associated with the development of MS in black and white youth.13 However, the precise mechanism underlying this relationship remains unclear.
In this review, we aim to explore the potential effects of FA on NAFLD through its interactions with metabolic pathways, DNA synthesis and methylation, oxidative stress in the liver and intestinal flora.
2 Folic ACID METABOLISM
Folic acid (FA) is a water-soluble B vitamin that is entirely absorbed from the diet. It plays a crucial role in cellular carbon metabolism and is primarily found in the intestinal tract of animals and dark green plants. Some FA is also produced by intestinal flora in humans. FA is also known as treticyl glutamic acid. When food-derived FA enters the small intestine, it needs to be hydrolysed into sphenoyl monoglutamate and diglutamic acid by bile and gamma-glutamyl carboxypeptidase. The absorption site is mainly in the proximal jejunum. FA is then absorbed by intestinal epithelial cells, where it is reduced to dihydrofolic acid (DHF) by dihydrofolate reductase (DHFR). Subsequently, DHF is further reduced to tetrahydrofolic acid (THF). The addition of a one-carbon (1C) moiety to THF by serine hydroxymethyltransferase (SHMT) generates 5,10-methylene-THF (5,10-MTHF). Under the action of methylenetetrahydrofolate reductase (MTHFR) and its coenzyme methylcobalamin, 5,10-MTHF acid is converted to 5-methylenetetrahydrofolate (5-MTHF), which is the biologically active form of FA. This reaction is irreversible.14 5-MTHF plays a pivotal role as a coenzyme in the transfer of carbon groups in vivo. These coenzyme FAs carry a variety of 1C units to participate in key biochemical reactions in the body, such as the synthesis of thymine nucleotides and purines, the methionine cycle, the production of S-adenosylmethionine synthetase (SAM), and transmethylation through methyltransferase (MT) to provide methyl for life activities (Figure 1). This process is essential for DNA and RNA synthesis and epigenetic regulation.15
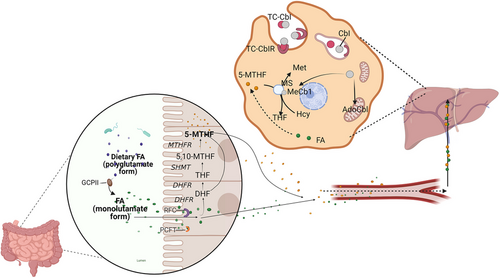
In the absence of THF, formaldehyde dehydrogenase and dimethylglycine dehydrogenase will produce formaldehyde from their respective substrates, which is then converted to formic acid by formaldehyde dehydrogenase. In the absence of THF, the 75% of dietary precursors of formic acid (serine, glycine, tryptophan, histidine, methionine and choline) will be converted to formic acid. Formic acid is an ending product of the oxidation of choline and amino acids and cannot participate in a carbon metabolic cycle.16, 17
Current research suggests that FA may play a role in regulating carbohydrate and lipid metabolism. Several epidemiological studies have found a negative correlation between serum FA levels and NAFLD in patients, as well as a positive correlation with homocysteine (Hcy) levels.9-11, 13, 18, 19 Studies on mice have also shown that maternal FA deficiency during pregnancy can increase obesity and IR in offspring, while providing FA to these offspring can improve both obesity and IR.20 Additionally, in vitro studies have demonstrated that FA can reduce triglyceride deposition by inhibiting fatty acid synthesis in primary chicken hepatocytes from NAFLD chickens.21 These findings suggest that FA may have potential as a treatment for NAFLD, potentially through the regulation of metabolically relevant gene epigenetic modification, Hcy metabolism, oxidative stress and gut microbiota.
3 THE MECHANISM OF FOLIC ACID IN NONALCOHOLIC FATTY LIVER DISEASE
3.1 Regulated nonalcoholic fatty liver disease related genes by DNA methylation
DNA methylation and the modification of the amino-terminal tail of histones are the primary forms of epigenetic modification observed in eukaryotic cells. These processes are crucial for maintaining intracellular homeostasis and function. DNA methylation occurs at the cytosine base of cytosine phosphate guanine (CpG) dinucleotides, resulting in the formation of 5-methylcytosine (5-mC).22 The DNA containing these methyl cytosine-rich CpG clusters is referred to as CpG islands. CpG islands are primarily located in gene regions associated with transcription initiation or promoters activity.22 In regulatory sites of genes, CpG islands are mostly unmethylated, whereas those present in non-regulatory regions are typically methylated.23 Methylation cytosine can modulate cell/tissue-specific gene expression by inhibiting transcription by blocking mRNA binding transcription factors or recruiting methyl-CPG binding domain proteins, such as methyl CpG binding protein 2 (MeCP2).24 The methylation of DNA is catalysed by DNA methyltransferases (DNMTs), of which DNMT1, DNMT3A and DNMT3B have been identified as having DNA methyltransferase activity.25 The methyl groups utilised by these enzymes are derived from SAM.25 Methyl donors found in food, such as FA, betaine and choline, are essential for SAM-mediated methyl transfer.26 FA acts as a catalytic substrate for transporting 1C units, while B12 serves as an important coenzyme for this process.26 In humans, in addition to the increased expression of three DNA methyltransferases, the hepatic expression of DNA non-acetylates ten-eleven translocation enzymes (TETs) is down-regulated.27 TETs are hydroxylases that catalyse the conversion of 5-methylcytidine (5-hmC) into 5-hydroxymethylcytidine. 5-hmC primarily mediates cell demethylation and leads to transcriptional genomic changes. Unlike 5-mC, the distribution of 5-hmC is tissue-specific and primarily observed in the liver, but the levels are greatly influenced by the cell state and external environmental factors27 (Figure 2).
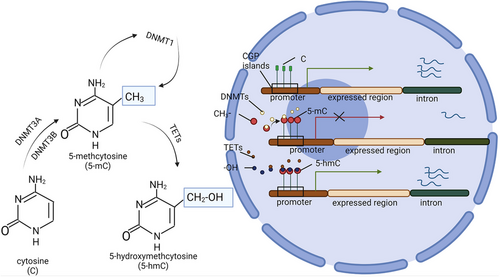
The close association between DNA methylation and the pathogenesis of NAFLD has been established. Lipid methyl-deficient mice exhibit diet-induced liver injury resembling human non-alcoholic steatohepatitis (NASH), along with abnormal histone modifications and alterations in liver DNMT1 and DNMT3A proteins.28, 29 Lai et al. reported that the level of total DNA methylation in the liver of both human and mouse NAFLD patients was reduced, with a decreasing trend observed in association with inflammation and disease progression.30-32 High global methylation of DNA and methylation of the promoter regions of lipid genes are crucial for maintaining normal metabolism by ensuring genomic stability and silencing abnormal lipogenic genes.33
3.1.1 Preclinical research
Loss of methyl donors, such as methionine, choline, FA, and betaine has been demonstrated to induce NAFLD, NASH and even HCC in rodents.34, 35 Conversely, supplementation with methyl donors can reverse overall DNA hypomethylation in the liver and attenuate NAFLD in rodent models.36-38 A diet low in methyl donors reduces hepatic SAM levels and causes CpG island demethylation of 164 genes involved in lipid and glucose metabolism, DNA damage and repair, apoptosis, liver development, fibrosis, remodelling and liver homeostasis, IR, and gene transcriptional regulation in mouse liver.39 Although methyl groups may be derived from a variety of substances, studies have found that a low-FA diet leads to decreased choline absorption.40 Furthermore, all methyl-metabolites in vivo require THF as a methyl vector, while vitamin B12 acts as a transmethylase coenzyme.40
FA influences the expression of genes in fatty acid synthesis. Pogribny IP and colleagues discovered that C57BL/6J mice on a methyl-deficient diet can lead to NASH, which are similar to the human. In comparison, DBA/2J mice, which are naturally deficient in histone H3 lysine 9 (H3K9) and H3 lysine 27 (H3K27) methylation, showed more severe NASH-specific pathological changes.29 Despite these differences, both DBA/2J and C57BL/6J mice showed comparable liver fat content when provided with a diet sufficient in methyl groups.29 Further investigations revealed significant demethylation across the genome and within repetitive elements in both DBA/2J and C57BL/6J mouse strains when fed a diet low in methyl groups, such as decreased levels of DNMT1, calmodulin-lysine N-methyltransferase (KMT), and PR/SET domain 2 (RIZ1). In addition, there was a reduction in the methylation of histone H3K27 and histone H4 lysine 20 (H4K20).29
Cordero et al. noted that high fat and sugar (HFS) diets modify the methylation status of genes implicated fatty acid synthesis in rats, including 1-fatty acylglycerol-3-phosphate O-acyltransferase 3 (AGPAT3), estrogen receptor 1 (ESR1), and fatty acid synthase (FASN). Supplemented with methyl donors can be reversal of fatty acid metabolism in HFS-diet rats through adjusted DNA methylation patterns within these genes’ promoter regions.41Salman et al. reported that rats on a high-fat diet (HFD) exhibited the histopathology feature of NAFLD, along with dysregulation of miR-21 and anomalous expression patterns of miR-34a and miR-122, as well as up-regulated expression of their target genes—HBP1, SIRT1 and SREBP-1c. Moreover, levels of TNF-α and adiponectin in these rats were also impacted. Notably, after administering a dose-dependent FA treatment, these alterations were significantly ameliorated.42
Regarding fatty acid oxidation, Pogribny et al. found that a diet low in methyl donors can lead to the hypermethylation and subsequent suppression of the carnitine palmitoyltransferase 1A (CPT1A) expression.43 Pooya observed that in rats, a deficiency in liver methyl donors is associated with impaired fatty acid oxidation, which can be attributed to the hypomethylation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and decreased interaction with its cofactors, estrogen receptor alpha (ESR-α) and hepatocyte nuclear factor 4 alpha (HNF-4α).44 In studies involving young mice on an HFD, an increase in hepatic dipeptidyl peptidase 4 (DPP4) expression was noted, along with a reduction in the methylation of CpG sites within four introns surrounding exon 2.45
Additionally, in the livers of rats with NAFLD, there was a higher level of methylation in the promoter regions of genes coding for microsomal triglyceride transfer protein (MTTP) and the low-density lipoprotein receptor (LDLR).46
These results imply that variations in dietary methyl content may act as a predisposing factor for the development of NAFLD.
3.1.2 Clinical research
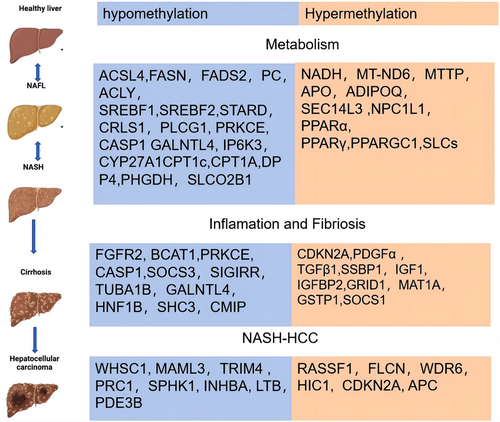
Many studies have confirmed DNA methylation changed in liver biopsy of patients with NAFLD45, 47-65 (Figure 3). A recent investigation revealed a marked reduction in global DNA methylation among 47 individuals diagnosed with NAFLD compared to a cohort of 18 lean subjects without NAFLD, as assessed via liver biopsy.30 Additionally, an inverse relationship was observed between DNA methylation levels and FA concentrations.56 Murphy et al. conducted an analysis on DNA methylation patterns using frozen liver biopsy samples from 33 NAFLD patients exhibiting early-stage fibrosis (F0-F1) and 23 individuals with advanced fibrosis (F3–F4). The study pinpointed 69 247 CpG sites with differential methylation, of which 76% demonstrated hypomethylation, while 24% showed hypermethylation in the context of progressive versus early fibrosis. It has been noted that global DNA methylation levels tend to diminish in concert with the escalation of liver inflammation, fibrosis and overall disease severity.49 Nevertheless, research into the epigenetic modifications following FA supplementation in NAFLD patients remains scarce. Drawing on insights from preclinical research, it is hypothesised that human epigenetic profiles may exhibit improvements with the introduction of a methyl-donor-enriched diet. Despite this, empirical studies investigating the effects of the FA treatment on methylation alterations in NAFLD patients are limited.
3.2 DNA methylation states are heritable and can be regulated by folic acid
Offspring of mothers exposed to an HFD showed abnormal liver fat metabolism. A Western diet (WD) with high fat and cholesterol induces hypercholesterolemia in mothers and predisposes offspring to NAFLD and metabolic diseases.
WD hypomethylated the promoter 2 and 3 of CD36, a fatty acid transport protein associated with fatty acid absorption, up-regulated the expression of CD36, and aggravated ApoB gene expression leading to NAFLD in male offspring rodents.66, 67 A high-fat and sugar diet in mother mice is associated with changes in acetyl-CoA carboxylase (ACACA), FASN, and phosphatidylinositol-4, 5-diphosphate 3-kinase (PI3K), resistin, and v-Raf-1 murine leukaemia virus oncogene homologous 1 (Raf1) and reduces insulin sensitivity and promotes lipid accumulation in offspring mice.68 A high-cholesterol diet during pregnancy and lactation in female apolipoprotein (Apo)E deficient mice resulted in their male offspring developing abnormal glucose tolerance, peripheral IR and fatty liver at 4 months. This may be related to the hypermethylation of CpG sites in a small part of the ApoB gene promoter regions.66
Human studies have shown that low FA intake during pregnancy is associated with a higher risk of obesity, IR, and gestational diabetes mellitus (GDM) in their offspring. A study of 24 maternal–infant pairs identified that methylation patterns changed at 993 CpG sites, with 116 (11.7%) increasing and 877 (89.3%) decreasing during pregnancy.69 CpG sites where methylation changed during pregnancy are associated with maternal peripheral blood concentration and 5-MTHF concentration in umbilical cord blood at delivery.69 These changes are associated with changes in the 1C units metabolite index during pregnancy. However, studies on DNA methylation changes in the offspring and the impact on glucose and lipid metabolism disorders caused by FA deficiency in the mother or father remain limited.
3.3 Normal DNA methylation requires the co-participation of folic acid and B12
However, FA supplementation is not without its drawbacks. Several studies, encompassing two human cohorts70, 71 and multiple rodent experiments,44, 72-75 have indicated that excessive maternal FA intake correlates with elevated blood glucose or IR)in progeny. This is posited to occur as excessive FA depletes vitamin B12, hindering the activation of FA to 5-MTHF and disrupting the methionine cycle—a phenomenon termed the ‘methyl trap’,15, 76 MacMillan et al. noted that, in rats with cobalamin deficiency, the distribution of 1C units from 10-MTHF shifts toward formic acid production, which fails to shifts methyl to generate SAM, leading to depletion of this critical methyl donor pool and subsequent 1C unit circulation dysregulation.77 Leclerc et al. discerned that a high-FA diet impairs the methylation of the lipolysis-stimulated lipoprotein receptor (LSR) gene, thereby affecting the expression of genes involved in cholesterol synthesis, transport or metabolism.78 Clinically, Yang et al. observed that elevated serum total FA and 5-MTHF levels are associated with a reduced prevalence of NAFLD and advanced fibrosis, while higher unmetabolised folic acid (UMFA) levels correlate with a greater prevalence of NAFLD,79 offering an explanation for the lack of FA deficiency observed in some NAFLD patients. Thus, to maintain a dynamic equilibrium, FA and B12 supplementation should be administered concomitantly.
To summarise, research on both animal models and human subjects has shown a significant connection between the progression of NAFLD and liver DNA methylation. Serum carbon metabolite levels have been found to reflect the histological type of NAFLD. As NAFLD progresses, genes involved in lipid synthesis, tissue repair and oncogenesis become hypomethylated, resulting in increased mRNA transcription. However, as liver fibrosis advances, methylation levels of anti-fibrotic genes and those associated with mitochondrial oxidative respiration increase, leading to decreased mRNA transcription.
Studies such as Lyall's genome-wide 5-hmC profiling have shown that although the overall level of 5-hmC is consistent, the distribution pattern varies and is influenced by environmental factors.80 Genes stimulated by an HFD tend to exhibit increased 5-hmC levels, while those suppressed by an HFD show decreased 5-hmC.80 This could account for the global hypomethylation observed in NAFLD patients. However, the tissue-specific nature of 5-hmC means that these regulatory effects of methylation may be region-specific,80 potentially explaining the overall decrease in DNA methylation while still observing some pro-fibrosis-related DNA methylation elevation in NAFLD.
Supplementation with 1C units such as FA may increase the global methylation level in NAFLD patients, presenting potential therapeutic benefits. Nevertheless, the number of clinical studies exploring the effects of FA supplementation on liver methylation in NAFLD patients is limited. Furthermore, while the heritability of DNA methylation states can be influenced by FA, the exact mechanisms involved are not fully understood. Excessive FA may even exacerbate NAFLD, potentially through the disordered transmethylation functions caused by vitamin B12 depletion. Therefore, the powerful management of NAFLD needs a balanced supplementation of both FA and B12.
4 FOLIC ACID REDUCE OXIDATIVE STRESS IN THE LIVER
4.1 Prevent oxidative damage caused by lipid accumulation
Currently, there is limited research on the antioxidant stress effects of FA in NAFLD. In animal studies, Huang observed a significant correlation between decreased liver FA concentration in rats with a low FA diet and increased liver lipid peroxides.72 Accumulation of lipid peroxides can trigger activation of pattern recognition receptors and unfolded protein responses (UPR) in hepatocytes and liver immunocytes, leading to lipotoxicity and liver inflammation in NAFLD. As previously mentioned, FA can enhance the metabolism of these lipids, thereby reducing oxidative damage caused by their accumulation.
4.2 Reduce oxidative damage caused by Hcy
In addition to the inflammation and oxidative damage caused by these lipids, individuals with NAFLD frequently exhibit increased Hcy concentrations. Hcy is an intermediary metabolite formed during methionine demethylation. Its primary metabolic routes include reduction through Betaine Hcy methyltransferase (BHMT) or Methionine Synthase (MS). A secondary pathway involves sulphur transformation, catalysed by Cystathionine beta-synthase (CBS)81 (Figure 4). Elevated plasma Hcy levels have been linked to dietary factors, particularly insufficient intake of FA, vitamin B6 and vitamin B12.82-84 Diets high in methionine can also raise plasma Hcy levels, and moderate hyperhomocysteinemia has been implicated in the progression of NAFLD in murine models. Additionally, Hcy-induced fatty liver has been demonstrated in primary mouse hepatocytes.85 Notably, Hcy levels inversely correlate with lean body mass (LBM).86 Excess methionine may be transformed into cysteine via CBS, although this process is slow, potentially due to reduced CBS mRNA expression levels in response to an HFD.85 Consequently, in the absence of adequate FA, the conversion of Hcy is limited, resulting in its significant accumulation.
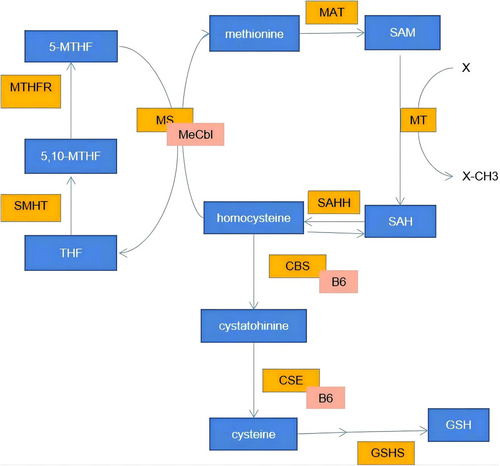
Hcy, as a potent oxidant, can trigger the release of reactive oxygen species (ROS) and c-reactive protein (CRP) via some signalling pathway such as MAPK and NF-κB. This stimulates the emission of cytochrome C and the activation of caspase 3, culminating in hepatocyte apoptosis.87 Prior research has revealed that Hcy impairs vascular endothelium and constitutes a risk factor for cardiovascular diseases.88 The toxicity to the vascular system may be attributed to tissue-bound and protein-bound Hcy,89 the phenomenon potentially linked to hepatic health as well. Hcy can form isopeptide bonds with lysine (Lys) residues in proteins, or due to its structural resemblance to cysteine (Cys), homocydtein can also interact with cysteine residues in proteins. These distinct post-translational modifications, known as “homocysteinylation” (Hcy-ylation), can disrupt protein structure or function, thus triggering UPR. Concurrently, the propensity of Hcy for oxidation results in abundant oxygen radical generation, causing endoplasmic reticulum or mitochondrial stress and furthering NAFLD progression.84 Misfold or unfold proteins activate UPR pathways, which then lead to the activation of the transcription factor SREBP-1c and the expression of genes associated with adipogenesis.85 Moreover, the UPR entails inhibiting mRNA transcription for fatty acid oxidation and transport proteins via Ire1-dependent regulated IRE1-dependent decay (RIDD), enhancing inflammatory and apoptotic signalling, promoting cellular apoptosis, and ultimately aggravating NAFLD.90
FA not only donates methyl groups via the methionine cycle but also plays a crucial role in mitigating the accumulation of its byproduct, Hcy. FA can maintain the function of syntaxin 17, a lysosome-binding protein, this control over Hcy levels is vital as it influences glutathione (GSH) biosynthesis and modulates the GSH transport system.91 Research by Tripathi and colleagues has demonstrated that FA contributes positively to the reduction of Hcy levels and the enhancement of GSH metabolism, and maintain the function of syntaxin 17 (a lysosome binding protein) by preventing homocysteylation of syntaxin 17.92 This process facilitates the elimination of abnormal proteins, thereby diminishing cellular damage and consequently attenuating inflammation and fibrosis in non-alcoholic NASH.92 In the cellular, Bagherieh and his team discovered that FA supports the scavenging of reactive ROS by lowering Hcy concentrations and curtailing the NF-κB signalling pathway in HepG2 cells, which ameliorates the inflammatory response triggered by palmitic acid.93
Methotrexate (MTX), an antagonist of FA, is extensively used in treating various conditions; however, its administration is often marred by hepatotoxicity as one of its most severe adverse effects. MTX administration results in concentration- and time-dependent rises in cytotoxicity and ROS production, alongside a reduction in mitochondrial membrane potential (MMP) in rat hepatocytes. Such deleterious impacts are attributable to MTX-induced oxidative stress and mitochondrial impairment, mediated by ROS generation and GSH depletion.77
These insights underscore the significance of FA in safeguarding hepatic cells and its role as an antioxidant, albeit indirectly.
4.3 Antioxidant effects of folic acid in other liver diseases
The antioxidant effects of FA have been documented in various liver diseases. Acute alcohol exposure leads to enhanced FA reabsorption and accumulation in the kidneys, while concurrently depleting FA concentrations in the liver. Such depletion coincides with elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), upregulated expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) isoforms 1 and 4, caspases 9 and 3, and reduced levels of anti-apoptotic metallopeptidase inhibitor 1 and GSH. Supplementation with FA reverses these effects, normalising liver FA content and markedly ameliorating indices of liver inflammation and oxidative stress. These observations imply that FA mitigates Hcy levels and enhances the endothelial nitric oxide synthase (eNOS) expression by upregulating tetrahydrobiopterin, thus circumventing nitrosative stress.94 Furthermore, FA's antioxidant properties have been noted in the context of acetaminophen-induced acute liver injury95 and arsenic-related liver damage.96
After a long-term ethanol exposure in pregnant and lactating maternal mice, elevated markers of lipid and protein oxidation and increased specific activity of glutathione reductase (GR) were detected in both the mothers and their 21-day-old offspring. Concurrently, levels of thiobarbituric acid reactive substances (TBARS) were augmented in the liver and pancreas. Notably, these oxidative stress markers were significantly diminished in the cohort administered an FA-enriched diet.97
In summary, FA has demonstrated efficacy in ameliorating oxidative stress within hepatocytes, potentially mitigating NAFLD. This protective mechanism may encompass the attenuation of lipid accumulation, reduction of Hcy concentrations, decreased peroxide formation, lowered incidence of protein Hcy-ylation, and subsequent suppression of UPR signalling pathways, thereby safeguarding hepatocyte integrity. These beneficial effects of FA have been similarly observed in other liver injury models, indicating that FA may serve as a broad-spectrum agent for alleviating diverse liver injuries through the mitigation of oxidative stress.
5 FOLIC ACID AND CANCER
5.1 Appropriate folic acid may prevent tumour formation and progression
Numerous studies indicate that FA is beneficial in preventing the initiation phase of cancer, it may paradoxically foster the progression and development of pre-existing tumours and subclinical carcinomas.98-103 For certain cancers, including rectal,104 cervical,105 lung106 and esophageal cancer,107 an inverse relationship has been observed between disease incidence and FA levels. A 2016 study among 312 HCC patients and 325 healthy controls reported significant reductions in FA levels in the HCC group.108 Lower serum FA concentrations at HCC diagnosis are independently linked to poorer survival outcomes, particularly in individuals with systemic inflammation or those who currently smoke.109-111 As hepatitis C virus (HCV) infection advances from chronic hepatitis to cirrhosis and eventually HCC, serum FA levels are seen to drop while Hcy levels rise, signifying its role in disease progression and carcinogenesis.103 The mechanism underlying the association between low FA and cancer may involve FA depletion leading to increased DNA strand breaks, uridine malformation, and repair defects, resulting in chromosome instability.112 Inappropriate binding of DNA methyltransferase to unrepaired damage or mismatches may promote local histone deacetylation, methylation, and regional hypermethylation, leading to tumour suppressor gene silencing.64, 98, 113 The low FA may lead to a compensatory increase of MTHFR. HCC cancer patients with higher MTHFR had a worse survival rate.112 However downregulated of MTHFR mRNA in HepG2-induced cell cycle arrest in G2/M that may promote nucleotide supply and assist cell defense against FA depletion-induced chromosome segregation and uracil misincorporation in the DNA. Moreover, FA deficiency may initiate the UPR, fostering a high ROS milieu. ROS is implicated in the transition of epithelial cells to mesenchymal cells (EMT) and in the genesis of cancer stem cells (CSCs).114, 115
5.2 Excess folic acid may promote tumour progression
The excessive intake of synthetic FA, from high-dose supplements or fortified foods, might hasten the expansion of precancerous lesions. A meta-analysis reported that prolonged FA supplementation heightened the risk of colon cancer.116 Diets abundant in FA significantly accelerated the progression of HCC in animals subjected to various carcinogens, such as diethylnitrosamine (DEN), an HFD, and carbon tetrachloride (CCl4).102, 117, 118 Animal studies have demonstrated that an FA-deficient diet lowers HCC incidence but raises the risk of liver fibrosis, suggesting a delay in HCC progression.102 FA assists in the integration of methionine and 1C metabolism, leading to HCC development through the methionine adenylyltransferase 2A (MTIα) metabolic pathway. Several metabolites produced in the FA cycle are vital for HCC growth. The FA cycle is a significant source of NADPH in all metabolic processes,119 with NADPH serving as an essential antioxidant in humans, indicating that the FA cycle is crucial for maintaining REDOX balance in HCC. Cells deficient in FA showed increased migration, invasion, apoptosis, necrosis and diminished CSC activity. In contrast, cells treated with excess FA exhibited heightened migration, invasion, cellular activity, CSC numbers, and reduced apoptosis and necrosis.120 The underlying mechanism may involve excessive FA depleting MTHFR, creating a “methyl trap” that interferes with normal DNA methylation regulation. Furthermore, FA supplementation can intensify the endoplasmic reticulum stress in tumour cells, enhancing their survival and metastatic potential.121
In conclusion, low FA levels may correlate with HCC and liver fibrosis development. Optimal FA concentrations can help cancer prevention, yet excessive levels could contribute to cancer promotion. FA quantification could be a useful prognostic tool for liver fibrosis and HCC. Some epithelial tumours exhibit higher densities of FOLR1 and FOLR2 on their surfaces,122 and the FOLR1-targeted drug Mirvetuximab Soravtansine-Gynx has been approved for FRα-positive, platinum-resistant ovarian cancer.123 Due to the increased expression of FA receptors on liver tumour cells, FOLR1 concentrations rise during the EMT process, boosting the FA uptake in HCC cells.124 Thus, modulating FOLR1 expression might also diminish the metastatic risk of HCC, but further investigation is required.
6 RELATIONSHIP BETWEEN INTESTINAL FLORA AND FOLIC ACID IN NONALCOHOLIC FATTY LIVER DISEASE
The intestinal microbiota plays a crucial role in food digestion, enhancing the uptake of dietary molecules and their metabolic byproducts into systemic circulation. Concurrently, it intersects with the mucosal immune system, facilitating the translocation of these compounds into the bloodstream through immune cells. This dynamic underscores the profound impact of the intestinal microbiota on diverse physiological processes. Compelling evidence from preclinical models and rigorous human studies indicates significant shifts in the gut microbiome of patients with NAFLD. For example, clinical epidemiologic studies have identified elevated levels of specific bacterial taxa, such as Proteobacteria, Enterobacteriaceae, Escherichia and Gammaproteobacteria, in subjects with NASH.125-127
In research compare the gut flora of NAFLD patients with that of obese counterparts, a heightened presence of gram-negative bacteria was observed in the NAFLD group. This was paralleled by a 20% surge in overall bacterial counts and a 24% decline in Firmicutes relative to healthy, non-obese controls. Notably, the decrease in Firmicutes included species known for synthesising health-promoting compounds such as the short-chain fatty acid (SCFA)-producing Lactobacillaceae, 7α-dehydroxylating Ruminococcaceae, and the rise in opportunistic pathogenic bacteria that produce LPS was also observed in patients with NAFLD.128 Patients with early and late NAFLD showed differences in microbiome characteristics. Faecal microflora analysis of NAFLD and NASH patients showed that Bacteroides abundance was significantly increased in NASH and F ≥ 2 patients, whereas Prevotella abundance was decreased,and Ruminococcus increased.126 Furthermore, children with NAFLD observed elevated levels Gammaproteobacteria and Prevotella alongside significant increases in ethanol concentration and distinct impacts on SCFA profiles.129 With disease progression, Bacteroidetes and several genera of Lachnospiraceae family, which have genes for deoxycholic acid (DCA) synthesis was increased, whereas the numbers of several beneficial microbes vulnerable to DCA's antimicrobial properties declined.130
The occurrence of non-obese NASH or advanced fibrosis (stages F2–F4) is linked to a distinct faecal mycobiome profile. This profile is marked by a heightened logarithmic ratio of Mucor sp. to Saccharomyces cerevisiae (S. cerevisiae) in individuals with NASH and advanced fibrosis. The C. albicans/S. cerevisiae was significantly higher in non-obese patients with NASH when compared with non-obese patients with NAFL or controls. Plasma anti-C. albicans IgG was increased in patients with NAFLD and advanced fibrosis.131 Research indicates that germ-free mice on an HFD maintain lower hepatic lipid concentrations than mice on a standard diet.132 Introduction of microbiota to the intestines of germ-free mice with hyperglycaemia and IR triggered the onset of NAFLD.133 The long-term antibiotic treatment has shown a reduction in small intestinal bacterial load and an enhancement in liver functionality.134 In a similar vein, short-term administration of rifaximin (an intestinal non-absorbable antibiotic) improved the liver function in patients with steatosis and NASH.134
To summarise, the gut microbiota–liver axis is crucial in the pathogenesis of NAFLD in both rodents and humans. For NAFLD patients, an increased intestinal microbial presence leads to heightened intestinal inflammation, permeability, and a greater likelihood of bacterial translocation. Such changes fuel systemic and hepatic inflammatory responses, thereby intensifying the severity of NAFLD. Consequently, strategies aimed at diminishing intestinal inflammation may offer therapeutic benefits for ameliorating the effects of NAFLD.
6.1 Folic acid may improve nonalcoholic fatty liver disease by reducing intestinal inflammation
Alterations in the gut microbiota can provoke liver inflammation by breaching the intestinal barrier, allowing microbial translocation and interactions with their metabolites as well as dietary elements, thereby contributing to steatosis. Patients with NAFLD and NASH exhibit compromised intestinal barrier integrity or increased permeability.133 Diets high in saturated fats have been shown to damage the intestinal epithelial cell layer, compromising the barrier function in animal models, while diets rich in fibre appear to enhance the microbial SCFA production and improve energy expenditure.135 Intestinal inflammation is intricately linked to methylation processes, with widespread DNA hypomethylation observed in the absence of methyl donors such as FA.136 FA insufficiency can also lead to uridine misincorporation during DNA synthesis, causing strand breaks and chromosomal damage.136 FA is capable of modulating chronic inflammation in inflammatory diseases by enhancing DNA methylation in the intestinal epithelium. Moreover, FA, along with other methyl donors, may downregulate pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumour necrosis factor (TNF), reduce C–C chemokine ligand 2 (CCL2) mRNA levels, and increase methylation at CpG sites within the IL-1β, serpin family E member 1 (SERPINE1), and interleukin-18 (IL-18) genes.136 FA not only bolsters intestinal DNA methylation, critical for proper gut development,137 but also supports the function of branched-chain amino acids (BCAAs) and mitochondria, which are implicated in obesity and gut microbiota interactions.138 Adequate FA levels are vital for sustaining forkhead box P3 (Foxp3) regulatory T cells (T-regs) in the colon. Dietary deficiency of FA leads to a marked decline in Foxp3 Tregs selectivity within the colon, heightening vulnerability to enteritis.139 Localised production of FA in the colon may also contribute to a reduced cancer risk in individuals with inflammatory bowel disease (IBD).139 The suppressive effects of FA and other methyl donors on the expression of IL-1β and TNF highlight the potential of FA in managing chronic inflammation within inflammatory diseases and improving intestinal permeability, predominantly through the mechanism of DNA methylation.140
6.2 The ability of the gut flora to produce folic acid is related to the type of gut flora
FA is predominantly absorbed in the small intestine, but FA transporters have also been identified in the colon,141 indicating that bacterially synthesised FA may be assimilable and contribute to host metabolic processes. It is estimated that humans assimilate approximately 322−396 μg of FA daily from the colon, with gut microbial synthesis accounting for at least 18%.142 Nonetheless, dietary FA intake does not always correlate with circulating FA levels, potentially due to genetic polymorphisms that influence FA metabolism and its enzymatic conversion.143 The synthesis of FA is linked to the composition and activity of the gut microbiota. Prior research has established that gut microbes can generate substantial quantities of FA and other B vitamins.144 In vitro studies have demonstrated that all strains of human-residential bifidobacteria (HRB) are capable of the FA production, in contrast to most non-HRB strains, except for B. thermophilum and B. longum ssp. suis.144 FA in human colon is mainly produced by Lactobacillus and Bifidobacteria, etc., while the abundance of FA producing bacteria in the intestinal flora of NAFLD patients is significantly reduced,128 which may explain the observed reductions in circulating FA levels.
Pompei et al. reported that Wistar rats on a diet supplemented with FA-producing probiotics and prebiotics exhibited elevated serum and hepatic FA concentrations.145 The utilisation of lactic acid bacteria (LAB) capable of producing FA as a natural alternative to synthetic supplements has been found to be more effective than the direct oral FA supplementation.146 A study employing VSL#3 (a compound probiotic capsule) with increased intestinal bifidobacterium in elderly individuals revealed sufficient FA levels, alongside reduced Hcy concentrations and diminished liver inflammation, under this probiotic treatment.147 These findings suggest that the composition of the gut microbiota can significantly influence circulating FA levels.
6.3 The ability of gut flora to produce folic acid is related to diet and internal environment
Bacterial FA biosynthesis can be enhanced through the administration of prebiotic fructans or by introducing FA-producing bacteria.145 Certain Lactobacillus strains are capable of synthesising FA by utilising para-aminobenzoic acid (PABA) as a precursor substrate in the medium, despite not being natural FA producers.142 While FA is predominantly present in vegetables and grains, a dietary shift from high-carbohydrate to low-carbohydrate (low-grain) in mice has been associated with elevated circulating FA levels and an apparent increase in faecal FA concentration. This could be attributed to the higher dietary protein content and reduced carbohydrate-dependent bacteria, potentially fostering the proliferation of FA-synthesising bacteria such as Streptococcaceae and Lactobacillaceae, thereby augmenting the bacterial FA production.145 These findings suggest that bacterial FA synthesis may be modulated by dietary factors. Rodent experiments indicate that bacterial FA biosynthesis can be stimulated by plant-derived fructose or by introducing FA-producing bacteria, culminating in increased FA concentration in serum or liver.145
In NAFLD patients, the gut microbiota's capacity to produce FA is compromised, leading to a deficiency of FA in the bloodstream, which might exacerbate intestinal inflammation. The resulting inflammation can disrupt the reductant–oxidant balance and further diminish bacterial FA synthesis,148 potentially contributing to a perpetuating cycle that exacerbates NAFLD progression. The production of FA in the gut is intrinsically linked to the bacterial community present, and both dietary and exogenous probiotic supplementation can stimulate FA production by modulating the gut microbiota. Although the role of dietary FA levels in shaping gut microbiota is an area of interest, research in this domain remains sparse. Future investigations should explore whether dietary or microbial FA supplementation could offer greater therapeutic benefits for NAFLD management than direct FA supplementation.
7 CONCLUSIONS AND FUTURE CONSIDERATIONS
FA plays a pivotal role in methyl metabolism and lipid metabolism in the liver. Supplementing with FA has been shown to alleviate hepatic steatosis in rodents. Beyond regulating lipid metabolism, FA also scavenges oxygen free radicals, modulates the activity of oxygen free radical-generating enzymes, restores the activity of antioxidant enzymes and reduces the secretion of inflammatory cytokines by immune cells. Proper FA intake may also prevent tumour formation and progression. In the gut, FA has the ability to improve intestinal permeability, mitigate systemic and liver inflammation, and reduce NAFLD (Figure 5).
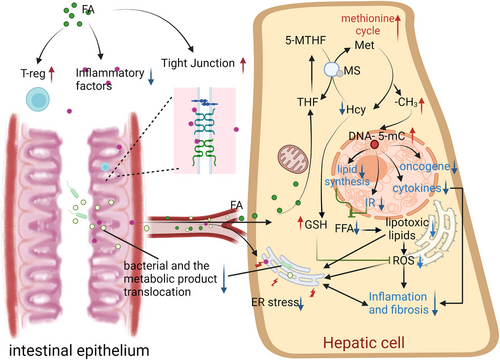
Despite the known beneficial effects of FA supplementation, oversupplementation of FA may mask B12 deficiency and may lead to liver inflammation increased and may promote tumour progression. In addition, studies have found that male offspring of female rats fed a diet high in FA during pregnancy have lower gut levels of butyrate (an SCFA that protects against obesity and related metabolic diseases).149 The decreased production of butyrate results in increased intestinal inflammation, increased gut permeability, endotoxemia and systemic inflammation.150 This suggests that excessive FA may also increase the risk of NAFLD by inhibiting intestinal butyric acid synthesis. In order to minimise adverse effects, appropriate clinical trials are necessary to determine the optimal dose of FA supplementation for specific populations, such as those with fatty liver disease or metabolic disease. The treatment method of restricting energy intake may lead to the inadequacy of FA and B12.151
In addition, metformin, an insulin sensitiser widely used in the treatment of NAFLD, IR and type 2 diabetes, has been found to inhibit the absorption of B12.152 Mahabir S et al. found the serum FA in the overweight postmenopausal women was lower than healthy individuals despite the adequate intake of FA.153 Therefore, the supplementation of FA and B12 in addition to the application of such patient may be beneficial to the NAFLD, and the effect of dietary structure changes or probiotic supplementation may be better than that of oral FA supplementation alone, but needed further verification studies.
AUTHOR CONTRIBUTIONS
Manuscript preparation: Bing'er Xu, Xinyu Yang, Hua Bian. Date collection & data analysis: Bing'er Xu, Xinyu Yang, Xiaopeng Zhu, Qiling Liu, Yuying Zhang, Miao Zhang, Chenmin Fan, Xilei Ban, Guligeina Aikebaier. Manuscript review & editing: Hua Bian. Funding acquisition: Hua Bian.
ACKNOWLEDGMENTS
We are funded by a grant from the National Natural Science Foundation of China (NCSF). Thanks are due to colleagues, collaborators, all researchers and volunteers who participated in the studies mentioned in this review.
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest.
ETHICAL APPROVAL
Not Applicable.




