Low-intensity pulsed ultrasound induces proliferation of human neural stem cells
The work was done in the School of Dentistry, University of Birmingham, Birmingham, UK.
Abstract
Background
Low-intensity pulsed ultrasound (LIPUS) has been highlighted as a potential therapy for tissue repair and regeneration. However, little is known about LIPUS effects on neuromodulation. This research was conducted to study LIPUS effect on the proliferation of human neural stem cells.
Materials and methods
The human SH-SY5Y neuroblastoma cell line was used as a neural stem cell model. The well-documented SH-SY5Y neurogenic protocol, which involves treatment with all trans-retinoic acid (ATRA) for 5 days and then brain-derived neurotrophic factor (BDNF) for 7 days, was used to synchronise the growth cell cycle to G1 phase of the cell cycle before proliferation testing. Subsequently, the neural stem cells were then treated with single or triple 20-min LIPUS exposures (Intensity ISATA: 60 mW/cm2, frequency: 1.5 MHz, pulse repetition: 100 Hz, and duty cycle: 20%). Cell proliferation was analysed using cell counting of β-tubulin and neurofilament medium-positive neural stem cells, Ki67-cell proliferation marker and metabolic-based assays (cell counting kit-8 and alamarBlue). The involvement of ERK signalling was investigated by quantification of phospho-ERK1/2 levels and cell proliferation with and without MEK/ERK inhibitor (U0126).
Results
The results show that LIPUS exposure(s) induced cell proliferation, as evidenced by an increase in the numbers of neural stem cells. ERK signalling is involved in LIPUS-induced neural stem cell proliferation, as evidenced by concurrent inhibition of LIPUS-induced phospho-ERK levels and cell proliferation in the presence of the MEK/ERK inhibitor.
Conclusion
This study provides original evidence that LIPUS can stimulate neural stem/progenitor cell proliferation. LIPUS may be suggested as a sole or an adjunct therapeutic application to promote the neural stem cell pool in stem cell therapies and tissue engineering approaches for nerve repair and regeneration for the management of traumatic nerve injuries and regenerative endodontic treatment.
1 INTRODUCTION
Low-intensity pulsed ultrasound (LIPUS) has gained interest over recent years as a safe, non-invasive and inexpensive clinical therapeutic tool to promote tissue regeneration and repair.1, 2 LIPUS has been approved as a therapy for delayed fractured bone union by the Food and Drug Administration (FDA).3 It has been advocated as a therapy for nerve repair and neuromodulation of rat nerve injuries.4-10 In this context, most LIPUS studies were conducted either on animals ‘in vivo’4-10 or on their cells ‘in vitro’,11-15 which cannot fully simulate human cells and genes hindering the translation of therapies to human.16-19 Furthermore, few studies have described LIPUS effects on human-derived neural stem cells.5, 20, 21 Hence, overall, little is known about the LIPUS effects on human neural stem cells.
As a preclinical step, in vitro human neural stem cells are required to investigate such a therapy. As it is prohibited to obtain primary human neural stem cells due to ethical concerns, human-derived neural cell line is an alternative option to study the therapeutic effects.22, 23 In this context, the human-derived SH-SY5Y neuroblastoma cell line24 is a basic and consistent neural model for neurological testing.25-27 SH-SY5Y is a subcloned cell line derived from a neuroblastoma of bone marrow in a 4-year-old girl,24, 28 which is widely used in neuroscience such as investigating Parkinson and Alzheimer's diseases,29, 30 neurotoxicity,31, 32 energetic neural vulnerability33 and being a control model for neurogenic induction of stem cells34, 35 and their neurotrophic effects.36, 37 However, the SH-SY5Y cell cultures lack mature neural features,33, 38, 39 and due to neuroblastoma ‘cancerous’ origin, the cells exhibit high proliferative rate40 with unsynchronised cell cycles,22, 41 which will negatively affect the reliability and reproducibility of the cell mitosis-related studies such as cell proliferation.42, 43 As the neurogenic induction of the SH-SY5Y cell line increases the neural characteristics and synchronises the cell cycle22, 38, 40 with low proliferation rate,38, 44, 45 which resembles the primary stem cells,40 this study was designed to be conducted on neurogenic-induced cells. In this context, the chosen neurogenic protocol using all trans-retinoic acid (ATRA) and brain-derived neurotrophic factors (BDNFs) has been proven to increase neural morphology and biomarkers,38, 39, 46 functionality-related genes47 and electrophysiological profile.48 Therefore, this study aimed to investigate the proliferative effect of LIPUS on neural stem cells established from SH-SY5Y.
2 MATERIALS AND METHODS
2.1 Cell culture and neurogenic preparation
SH-SY5Y neuroblastoma cells (ATCC CRL-2266) were used at passages of 18–22 and cultured in Dulbecco's modified Eagle's medium/mixture F-12 Ham (DMEM/F-12) (Sigma-Aldrich). The DMEM/F-12 was supplemented with 10% foetal bovine serum (FBS) (Biosera) and 1% penicillin-streptomycin (100 I.U./mL) (Sigma-Aldrich). Subsequently, the SH-SY5Y cells were kept in an incubator at 37°C and 5% CO2 (Heracell 150i, Thermo Scientific). The media changes were performed every 3 days till the cultures reached approximately 80% cell confluence. As the cell cultures exhibit a mixture of adherent (S-type) and detached (N-type) cells,22 the detached cells in the media were transported to a 50-mL tube before detaching the adherent cells by 0.25% trypsin/ethylenediaminetetraacetic acid (Trypsin/EDTA) in the incubator for 1–2 min. The mixture was then neutralised by complete growth media (10% FBS-supplemented media). The detached cells by Trypsin/EDTA were combined with the cells in the 50-mL tube, followed by centrifugation (220 × g, 3 min). The resultant cell pellet was resuspended in 1 mL of the FBS-supplemented media. The cell counting was then performed for cell seeding. A cell density of 10,000/cm2 was seeded in each well of a collagen-I-coated 6-well plate (Gibco A1142801, Thermo Fisher Scientific) for metabolic-based cell viability and proliferation assays or on laminin-coated coverslips (72298-08, Electron Microscopy Sciences, CN Tech Lab Supplies) for immunostaining assays for cell counting and Ki67-cell proliferation indicator. In addition, the cells (10,000/cm2) were seeded in T25 flasks (25 cm2) for ELISA quantification of ERK 1/2 (phospho-p44/42 MAPK) proteins in which the culture surfaces were un-coated to ensure the purity of the quantified proteins. Subsequently, the cells were incubated overnight. The cells were then cell-cycle synchronised using the well-documented neurogenic protocol described by Encinas et al.38 for 12 days (see the flow chart of the neurogenic protocol and LIPUS treatment in Figure 1A). Briefly, the cells were treated with ATRA (R 2625, Sigma-Aldrich) in the presence of 10% FBS for 5 days and then treated with BDNF (SRP3014, Sigma-Aldrich; 78005, STEMCELL Technologies) in serum-free media for 7 days. After that, the cells were replenished with fresh BDNF-supplemented serum-free media before starting the LIPUS experiments. All plasticware were purchased from Thermo Scientific, and all materials from Sigma-Aldrich, unless otherwise stated.
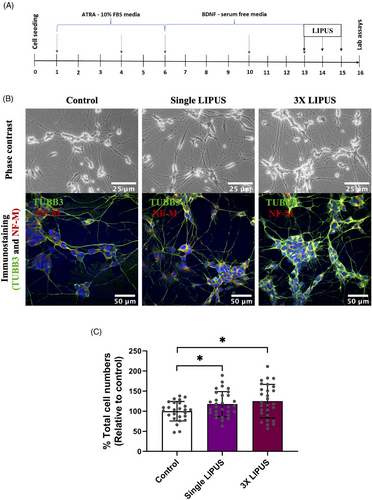
2.2 LIPUS set-up
The LIPUS device (Osteotron IV) was used to deliver LIPUS (the intensity ISATA: 60 mW/cm2, frequency: 1.5 MHz, pulse repetition: 100 Hz and duty cycle: 20%) for 20 min as previously described with some modifications.49 The cell culture wells were placed on the top of the transducers with a thin coat of ultrasound transmission gel (Aquasonic 100, Parker Laboratories). To avoid LIPUS wave interference between the LIPUS groups, the only corner wells on each side of 6-well plate were used, whereas the middle wells were untreated and filled with phosphate-buffered saline (PBS). Control groups were cultured in a separate 6-well plate and treated the same way as the LIPUS plates but without actual LIPUS exposure. Thus, the cell cultures were treated with LIPUS stimulation, and the control cell cultures were treated with sham stimulation (LIPUS machine was turned off). The LIPUS treatment was either single or triple exposures (once per day for 3 successive days), in which the treatment was completely conducted in the incubator (37°C, 5% CO2). After the 3-day LIPUS exposure, the analyses were conducted (Figure 1A). All described steps were gently performed because the SH-SY5Y-derived neural stem cells are small in size and easily disturbed.
2.3 Immunocytochemistry
The cell cultures were fixed with 4% paraformaldehydefor 10 min. The cells are then only permeabilised for anti-Ki67 intracellular antibody with Triton X-100 (0.5% prepared in PBS) for 10 min. After that, the cell cultures were blocked with blocking solution (10% goat serum, 3% bovine serum albumin [BSA] and 0.1% Triton X-100 prepared in PBS) for 1 h. The cell cultures were then incubated with the diluted primary antibody overnight at 4°C (diluent solution: 3% BSA prepared in PBS with the addition of 0.05% tween-20; see the dilutions and antibodies in Table 1). Negative controls were incubated with blank diluent solution (no antibody addition). Subsequently, all cell cultures were incubated for 1 h with the diluted secondary antibody in a light-protective container (the dilutions and antibodies are presented in Table 1). Finally, the cell cultures were mounted on microscopic slides by a mounting agent with DAPI (Abcam) for nuclear staining. These slides were kept in the light-protective container at 4°C until microscopic scanning using a confocal microscope (Zeiss LSM 700). There was washing step with PBS (3 × 10 min) after all previously mentioned steps except between blocking and primary antibody placement.
| Antibody | Type | Host species | Description | Dilution | Supplier | Product number |
|---|---|---|---|---|---|---|
| Anti-βIII-tubulin (2G10) | Primary | Mouse | Monoclonal | 1:400/500 | Abcam/Sigma-Aldrich | ab78078/T8578 |
| Anti-160 kD neurofilament medium | Primary | Rabbit | Polyclonal | 1:1000 | Abcam | ab9034 |
| Anti-Ki67 | Primary | Rabbit | Polyclonal | 1:200 | Abcam | ab66155 |
| Alexa Fluor 555 | Secondary | Goat | Polyclonal | 1:500 | Abcam | ab150078 |
| Alexa Fluor 488 | Secondary | Goat | Monoclonal | 1:400 | Invitrogen, Thermo Fisher Scientific | A20181 |
2.4 Cell counting using image analysis
The images of the cells immunostained for the neural markers (β3-tubulin alone or β3-tubulin and neurofilament medium) and proliferation marker (Ki67) were used to count total cell number and Ki67-labelling cells per image of each experimental group. The DAPI-stained nuclei of the cells labelled with neural markers were counted per experimental image using a Cell Counter plugin in Fiji-ImageJ software (https://imagej.nih.gov/ij/plugins/cell-counter.html). The relative percentage was then calculated. Similarly, Ki67-labelling cells and total cell number were counted using the Cell Counter plugin. The proliferation ratio of Ki67-immunopositive cells was then calculated among the total number of DAPI-labelled cells per image. The mean of the cell numbers was computed from random images of the experimental groups.
2.5 Metabolic-based cell viability and proliferation assays
The cell counting kit-8 (CCK-8) and alamarBlue (AB) assays are metabolic-based assays which were used to estimate cell numbers. These assays were validated for cell proliferation using known cell numbers seeded per well of 96-well plate (39,062.5, 78,125, 156,250 and 312,500/cm2). The cells were then incubated at 37°C with 5% CO2 for 6 h to allow cell adherence to the culture surfaces. The assays were then performed according to manufacture instructions. Briefly, cell cultures were incubated either with a 10% final concentration of CCK-8 or AB in blank DMEM/F12 media without any supplements at 37°C with 5% CO2 for 3 or 3.5 h, respectively. The absorbance was then read using a plate reader (BioTek Instruments; Tecan, ELx800; AB: wavelength of 570 and 600 nm; CCK-8: wavelength of 450 and 460 nm as reference filter).
2.6 ERK1/2 (p44/42MAPK) signalling
The ERK/MAPK signalling pathway has been involved in LIPUS-promoted cell proliferation of some cell types.5, 50-52 It was of interest to study whether the ERK/MAPK pathway was also involved in LIPUS-promoted neural stem cell proliferation. As both LIPUS exposures (single and 3× LIPUS) were effective in inducing cell proliferation, the single 20-min LIPUS exposure was selected for this investigation.
The cell cultures were supplemented with or without 10 μM U0126 (MEK/ERK inhibitor, Cell Signaling Technology) as previously described51, 53 for 1 h before single LIPUS or sham exposure. The cells were then directly lysed for ELISA quantification of ERK 1/2 (phospho-p44/42 MAPK) or further 48-h incubated for the Ki67-immunostaining assay, which is previously described in immunocytochemistry section. The cell lysis and ELISA work were carried out according to the manufacturer's instructions (PathScan Phospho-p44/42 MAPK: Thr202/Tyr204, Sandwich ELISA Kit, Cell Signaling Technology). Briefly, the cell cultures were immediately rinsed twice with PBS and lysed with lysis solution for 5 min incubated on ice. The lysis solution was supplemented with a protease inhibitor (1 mM phenylmethylsulphonyl fluoride, Sigma-Aldrich) to prevent protein degradation. Subsequently, the lysed cell cultures were sonicated (∼30 s) and centrifuged at 14,000 rpm using cold centrifuge (4°C) (Centrifuge 5415R, Eppendorf) for 10 min. The cell lysates were then kept at −80°C until ELISA work.
The Phospho-p44/42 MAPK-coated 96-well plate and the lysates were defrosted at room temperature. Lysate of 100 μL was added per well (duplicate wells per experimental group), sealed and kept overnight at 4°C. A volume of 100 μL detection antibody, HRP-linked secondary antibody and TMB solutions were then sequentially added, sealed and incubated for 1 h, 30 min and 10 min at 37°C and 5% CO2, respectively. The wells were washed with wash buffer (four times) using an automated well-plate washer (BioTek) after each step. The assay reaction was ended by 100 μL stop solution per well. The absorbance was then read at 450 nm using a microplate reader (Spark, Tecan). The data were normalised to total cell numbers and then expressed as relative percentages to controls.
2.7 Statistical analysis
All experiments were repeated at least twice with two to four technical replicates per experimental group. Data analysis was performed using IBM SPSS Statistics (version 26). The normality of the data was assessed by Kolmogorov–Smirnov tests. The statistical comparative test was then decided either one-way ANOVA followed by an appropriate post hoc test based on the findings of the homogeneity test of variances for parametric ‘normal distributed’ data or the Kruskal–Wallis test followed by the Bonferroni post hoc test for nonparametric ‘non-normal distributed’ data. These were the main statistical tests to compare between the experimental groups unless otherwise stated. The statistical significance level was set at p ≤ .05. The data were shown as mean ± standard deviation unless otherwise stated. The bar graphs were created using GraphPad Prism 9.
3 RESULTS
3.1 LIPUS increased cell numbers
Phase contrast and immunostaining images of the experimental groups clearly demonstrated that the LIPUS-induced groups exhibited a higher neural stem cell population compared with control group (Figure 1B). The immunostained images (β-tubulin [TUBB3] and neurofilament medium [NF-M] immunopositive) were chosen for cell counting using the cell counter, Fiji-ImageJ analysis software. The percentage mean average for the control group was 100 ± 24.27, whereas the single LIPUS and 3× LIPUS groups demonstrated a mean average of 117.81 ± 30.96 and 125.08 ± 41.65, respectively. Statistically, the data revealed that a significant difference was evident in cell numbers between the groups (Figure 1C; one-way ANOVA test, F-statistics (df): F (2.81) = 4.046, p = .021). LIPUS-induced groups demonstrated higher statistically significant cell numbers than the control group (Figure 1C; p = .050 for control vs. single LIPUS and p = .024 for control vs. 3× LIPUS). The 3× LIPUS group demonstrated relatively higher cell numbers compared with single LIPUS group; however, this difference was not significant (Figure 1C: p = .734). These findings suggest that LIPUS promoted cell proliferation. Next, we explored whether the LIPUS-induced increase in cell numbers was reflected by overall increase in viable cell numbers of the cell population.
3.2 LIPUS increased viable cell numbers
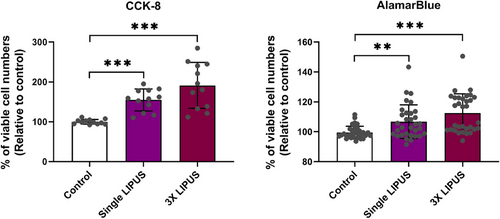
Two ‘metabolic’ viability assays (CCK-8 and AB) were validated, and data showed a linear relationship (R2 coefficient >.9) between the cell seeding numbers and absorbance levels of both metabolic-based assays. This means the increase in absorbance values reflected the increase in viable cell numbers per experimental group (Supporting information file). Consequently, both metabolic assays demonstrated that LIPUS significantly increased the viable cell numbers (CCK-8 assay: one-way ANOVA F ratio (df) = 18.607 (2,33), p < .001; AB assay: Kruskal-Wallis test - test statistics (df) = 24.801 (2), p < .001). The single LIPUS group (20-min exposure) demonstrated significantly greater levels of cell-count absorbance than control group (Figure 2; CCK-8 assay: p < .001and AB assay: p = .009). The 3X LIPUS group (once per day for 3 successive days) resulted in a further significant difference in levels of the cell-count absorbance compared with the control/sham group (CCK-8 and AB assays: p < .001). The 3× LIPUS group was also higher than single LIPUS group assessed by both assays, but there was no statistically significant difference (Figure 2; CCK-8 assay: p = .147 and AB assay: p = .138) underscoring the image analysis data of the immunostained cells. Next, we examined whether the increase in cell numbers was in fact caused by increase in cell proliferation.
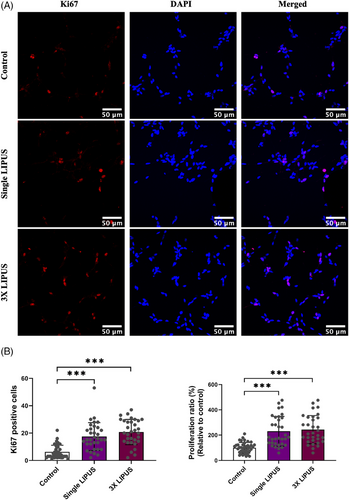
3.3 LIPUS-increased neural stem cell numbers were due to increased cell proliferation as evidenced by Ki67-cell proliferation indicator
The immunocytochemical data demonstrated that both LIPUS groups exhibited a greater number of Ki67-positive nuclei cells than control group indicating that the increase in neural stem cells is due to an increase in cell proliferation. However, the 3× LIPUS group exhibited the greatest Ki67-positive cells (Figure 3A). The statistical analysis demonstrated that there is a statistical difference in the number of Ki67-positive nuclei cells (Kruskal–Wallis test – test statistics (df) = 46.509 (2), p < .001). In line with the previous data, the numbers of Ki67-positive nuclei cells in the LIPUS groups were statistically higher than control group (Figure 3B: adjusted p value <.001). The 3× LIPUS group had the greatest numbers of Ki67-positive cells, albeit not statistically different compared with the single LIPUS group (adjusted p = .588). The proliferation ratio (Ki67-staining index) also revealed a statistically significant difference between the groups (Kruskal–Wallis test – test statistics (df) = 44.842 (2), p < .001). Both single and 3× LIPUS groups demonstrated significantly greater percentage of Ki67-positive cells than control group (Figure 3B: adjusted p < .001). No significant difference was detected between the single and 3× LIPUS groups (adjusted p = 1). Next, it was of interest to examine if ERK/MAPK signalling was involved in the LIPUS-promoted neural stem cell proliferation.
3.4 ERK/MAPK signalling was involved in LIPUS-induced neural stem cell proliferation
The immunostaining demonstrated that the increase of Ki67-lablelled cells in the LIPUS-induced groups was noticeably reduced after U0126 pre-treatment (MEK/ERK inhibitor). However, the LIPUS with U0126 group still displayed greater Ki67 levels than control groups with and without U0126 (Figure 4A). Statistically, the data confirmed that there is a highly significant difference in Ki67-stained cell numbers (one-way ANOVA: F ratio (df) = 22.697 (3,75), p < .001) and in proliferation ratio (one-way ANOVA: F ratio (df) = 33.185 (3,75), p < .001). In this context, the LIPUS-induced group exhibited statistically greater Ki67-positive nuclei and proliferation ratio than LIPUS with U0126 group (Figure 4B, Ki67 numbers: p = .002; proliferation ratio: p = .050) and control groups (p < .001). In contrast, no statistical difference in the Ki67-cell numbers or proliferation ratio was detected between the control groups with and without U0126 (Figure 4B, Ki67 numbers: p = .969; proliferation ratio: p = .658). Surprisingly, the LIPUS with U0126 group demonstrated significant greater Ki67-cell numbers and proliferation ratio than the control group (Figure 4B, Ki67 numbers: p = .041; proliferation ratio: p < .001), and control with U0126 group (Ki67 numbers: p = .006; proliferation ratio: p < .001).
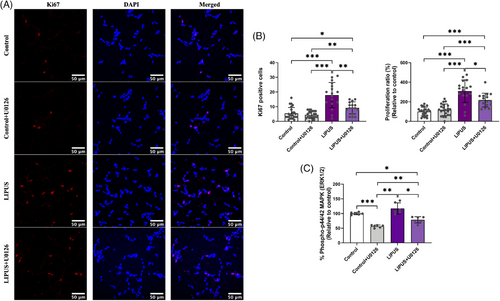
The ERK/MAPK ELISA revealed similar results to the Ki67 staining, demonstrating that the LIPUS-induced groups exhibited the highest phospho-ERK1/2 levels among the tested groups, and the U0126 pre-treatment lowered the levels of the phospho-ERK1/2 proteins (Figure 4C). Statistically, a high significant difference between the tested groups was detected (one-way ANOVA test, F-statistics (df) = 32.079 (3,20), p < .001). Although LIPUS-induced group showed a noticeable increase in phospho-ERK1/2 proteins compared to the control group, this increase was not statistically different (Figure 4C, p = .247). However, the significant upregulated levels of phospho-ERK1/2 proteins were detected in the LIPUS-promoted group compared with the control with U0126 (p = .002), and LIPUS with U0126 (p = .012) groups (Figure 4C). Likewise, the U0126-treated groups (control with U0126 and LIPUS with U0126) showed a significant reduction in levels of phospho-ERK1/2 proteins compared to the control group (control with U0126 vs. control: p < .001; LIPUS with U0126 vs. control: p = .013). Notably, the LIPUS with U0126 group resulted in significantly greater phospho-ERK1/2 levels than control with U0126 group (Figure 4C, p = .010). Taken together, these data indicate the involvement of the ERK/MAPK signalling and possibility of involvement of other upstream regulator(s) ‘beside to MEK1/2 regulator’ for ERK phosphorylation in the LIPUS-induced neural stem cell proliferation.
4 DISCUSSION
The present study demonstrated that LIPUS exposure induces cell proliferation of neural stem cells established from human-derived SH-SY5Y cells. This study also shows that the ERK1/2 signalling may play a significant role in the proliferative process of LIPUS on the neural stem cells. These novel findings imply that therapeutic LIPUS can trigger the cell proliferation of human neural stem cells.
The data of the cell counting and viable cell number assays corresponded with the Ki67-cell proliferation marker54 underscoring the cell proliferative effect of LIPUS on the neural stem cell cultures. The findings are consistent with other LIPUS studies on various cell types as assessed by cell viability assays13, 20, 51, 55-58 and proliferation indicators,20, 51, 52, 55, 59 which reported a significant increase in cell numbers and/or higher cell viability after LIPUS treatment. Similarly, the present study agrees with results of previous studies using various neural stem cell types such as the cell viability of SH-SY5Y cells60 and the cell proliferation of iPSC-derived and rat neural stem cells.15, 20 However, there are differences in LIPUS settings, in particular, the effective LIPUS intensity dose to increase cell viability and/or proliferation of neural stem cells. For example, Guo et al.60 reported that LIPUS induces a significant increase in the cell viability of SH-SY5Y cells at lower intensity dose (15 mW/cm2) compared with the present study (60 mW/cm2). In contrast, Lv et al.20 and Wu et al.15 reported that higher intensities of LIPUS (500 and 69.3 mW/cm2) compared with the present study (60 mW/cm2) produce a significant increase in cell proliferation of iPSC-derived and rat neural stem cells. There are also differences in the other LIPUS parameters such as much higher pulse repetition rate (1 KHz) in the study of Guo et al.60 and lower frequencies (1 MHz) in the study of Lv et al.20 and Wu et al.15 compared with the present study (pulse repetition rate: 100 Hz; frequency: 1.5 MHz). Hence, the effective LIPUS intensity dose may vary according to other LIPUS parameters used. In other words, when high pulse repetition or frequency is used, lower intensity is selected and vice versa. Regarding the intensity dose (60 mW/cm2) used in the present study, it has also been reported as an effective dose that induces cell proliferation and/or regeneration of cartilage49, 61 and muscle cells.62 Overall, regardless of the differences in cell types and LIPUS settings, the consistency in the results of the studies suggests that the LIPUS can promote neural stem cell proliferation.
Considering the agreement in the increase of the viable cells assessed by metabolic assays, it indicates that LIPUS might stimulate metabolic activities which subsequently result in cell proliferation. This interpretation agrees with Huang et al.63 who reported that the metabolite compositions and genes have a significant role in regulating cell proliferation induced by LIPUS. Another interpretation of the LIPUS-induced cell proliferation was highlighted by He et al.,64 Xie et al.57 and Ling et al.51 who reported that LIPUS drives the stem cells from the ‘stationary phase’ into the ‘proliferative phase’ of the cell cycle, resulting in cell proliferation. Similarly, Hu et al.58 showed that LIPUS produced greater cell viability of cardiomyocytes through entering the active cell cycles ‘S and G2/M phases’ from the stationary phase ‘G0/G1 phase’. In this context, the SH-SY5Y-derived neural stem cells used in this study have been reported to be arrested in the ‘stationary G1 phase’ of the cell cycle after neurogenic induction.38 Interestingly, the G1 phase can be stimulated(s) to re-enter the active cell cycle and proliferate or lengthen the phase for neurogenesis, as reported in some studies.65-67 In addition, the SH-SY5Y-derived neural stem cells can still proliferate after neurogenic induction, but in much lower rate compared with un-induced cells.38, 44, 45 Hence, the proliferated cells in this study might be either pre-existing neural stem cells or activated neurogenic-induced neural stem cells.
The results demonstrated that the MEK/ERK inhibitor (U0126) significantly reduced the neural stem cell proliferation (Ki67-labelled cells) and ERK phosphorylation induced by LIPUS, which indicates involvement of ERK/MAPK signalling. These results agree with some studies that highlighted the role of ERK signalling in LIPUS-promoted cell proliferation.5, 50-52 In this context, it would be interesting to investigate the dose-dependent effect of the MEK/ERK inhibitor (U0126) to elucidate whether certain inhibitor concentration(s) can fully block LIPUS-induced ERK phosphorylation and cell proliferation (Ki67-labelled cells) without affecting the cell proliferation of LIPUS-untreated cells. Unexpectedly, the control group with MEK/ERK inhibitor demonstrated a decrease in phospho-ERK levels, but this decrease was not associated with any significant change in Ki67-cell proliferation. These results indicate the phospho-ERK decrease of the controls is not related to the cell proliferation. As the ERK signalling pathway is central for multiple biological processes,68 it justifies the decrease in phospho-ERK levels of the controls with MEK/ERK inhibitor. Furthermore, the LIPUS with the MEK/ERK inhibitor group showed significantly higher cell proliferation and ERK phosphorylation than the control with MEK/ERK inhibitor group. This suggests that the LIPUS-promoted cell proliferation and ERK phosphorylation are partially inhibited by MEK/ERK inhibitor, and other upstream signalling inducer(s) may be involved in this process. This interpretation is consistent with Zhou et al.50 who reported that there is another upstream regulator (ROCK/Rho) besides the MEK regulator triggering ERK phosphorylation and cell proliferation activated by LIPUS. In addition, it has been highlighted that other regulators rather than the classical ‘MEK–ERK cascade’ can trigger ERK/MAPK signalling.68, 69 Taken together, LIPUS may stimulate more than one upstream regulator inducing ERK signalling in SH-SY5Y cells. Moreover, as multiple signalling pathways are elicited by LIPUS such as PI3K-Akt and ERK/MAPK,51 ERK, JNK or JNK and p38,52 ERK, JNK and p38 MAPK,70 PI3K-Akt,59 notch,15 GSK-3β/β-catenin,13 and PI3K-Akt and JNK MAPK,55 Ca 2+ signalling,11 further investigations are required to reveal all involved signalling pathways induced by LIPUS and their interplays in the neural stem cells. In this context, advanced techniques such as proteomic quantification71, 72 or genomic techniques73, 74 can be used to reveal all involved pathways, their regulators and their interplays.
Regarding possible clinical translation of the present research, LIPUS may be effective as a sole therapeutic tool or combined with another therapeutic approach such as microbubbles, which has been reported to enhance the LIPUS effects on cell proliferation and differentiation75-77 and neovascularisation,78 for stem cell therapy and tissue engineering approaches in the regenerative medicine. More specifically related to the present study, the LIPUS-induced cell proliferation of neural stem cells would benefit nerve repair and regeneration approaches like induction of nerve supply for regenerative endodontic treatment and management of traumatic nerve injuries.
Despite the best efforts, this study has a potential limitation. Although the SH-SY5Y-derived neural model showed neural features and simulated neural stem cells, it may not accurately represent the primary human neural stem cells and their physiological conditions. Thus, further in vivo studies are required to evaluate LIPUS effects on human neuromodulation.
The present study demonstrates that LIPUS exposure is able to stimulate cell proliferation of the human neural stem cells, which seems to be in part mediated by the ERK/MAPK signalling pathway. LIPUS may be a suitable non-invasive therapeutic tool to stimulate neural stem cell expansion in nerve repair and regeneration. Further studies are required to reinforce the clinical translation of the application(s) of LIPUS.
AUTHOR CONTRIBUTIONS
Conception; methodology; data interpretation; Final approval of the version: Arwa A. Al-Maswary, A. Damien Walmsley, Paul R. Cooper and Ben A. Scheven. Investigation and data collection; data analysis; visualisation (images and graphs); writing – drafting: Arwa A. Al-Maswary. Supervision; writing—revision and editing: A. Damien Walmsley, Paul R. Cooper and Ben A. Scheven.
ACKNOWLEDGEMENTS
Special thanks to Gay Smith, Michelle Holder and Helen Wright for their technical assistance and advice during the laboratory work of this study at School of Dentistry, University of Birmingham. Authors wish to thank Dr Huzaimi Haron, PhD student from School of Pharmacy, University of Birmingham for donating SH-SY5Y neuroblastoma cell line.
CONFLICT OF INTEREST STATEMENT
The authors declare that no conflicts of interest exist.
FUNDING INFORMATION
IsDB Merit Scholarship, IDB/Grant No. 600031755; School of Dentistry—University of Birmingham, Grant No. GAM2271
ETHICS STATEMENT
Not applicable
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.




