Progress of Actinidia chinensis Planch. root extract (acRoots) in cancer immunity
Abstract
Actinidia chinensis Planch. root extract (acRoots) is a famous Chinese traditional medicine used in the treatment of a variety of diseases, such as tumours and cardiovascular diseases, etc. However, its therapeutic mechanisms remain unclear. In this review, the biological features of acRoots and its chemical compositions are explored, including polysaccharides, triterpenes, phenols, flavonoids and so on. The pathogenesis of cancer involves not only genetic factors but also the microenvironment. Different types of immune cells, such as T cells, macrophages and neutrophils, play an essential role in cancer development, and acRoots was found to take part in modulating cancer immunity. Therefore, this article summarises its role in cancer immunity and unmasks the potential underlying mechanisms by incorporating our research on cancer immunity.
1 INTRODUCTION
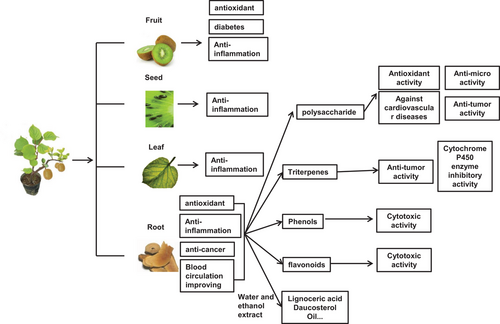
Actinidia chinensis Planch is commonly known as a native fruit of China, the kiwifruit. All parts of the plant, including its fruits, seeds, vines, leaves and roots, have been applied in traditional Chinese folk medicine.1 The fruit of Actinidia chinensis Planch (FACP) reportedly has beneficial properties against diabetes. Cholamreza Mohajeri et al. discovered that FACP significantly reduced the surface area of neuropathic diabetic foot ulcers by promoting collagen and granulation tissue formation and angiogenesis.2 Another study reported that the FACP exerted antioxidant and anti-inflammation effects on type 2 diabetes mellitus (T2DM) patients by upregulating miR-424, controlling the higher levels of superoxide dismutase and glutathione (GSH) and inhibiting the secretion of pro-inflammatory cytokines, such as interleukin-1 (IL-1) and interleukin-6 (IL-6).3 In addition to the fruit, Deng et al. found that the seed compound extract also exhibited strong anti-inflammatory activities by suppressing IL-1 and tumour necrosis factor-alpha (TNF-α).4 Furthermore, Gyoung-Deuck Kim et al. also found that the leaf compound extract of kiwifruit contained caffeoylthreonic acid and salvianolic acid A, which possessed anti-inflammatory effects.5 The root of Actinidia chinensis Planch (acRoots) has a bitter taste. The extract of the root was also known to have various medical effects, such as improving blood circulation, anti-oxidant, anti-inflammatory and anti-cancer properties.6 This review briefly outlines the biological features of acRoots, its chemical compositions and functions, especially in the context of cancer immunity.
2 CHARACTERISTICS OF THE ROOT OF Actinidia chinensis PLANCH
2.1 Biological features
The roots of Actinidia chinensis Planch, called Tengligen, are widely used in Chinese folk medicine and herbal compounds.7 Its main root is not developed, but the secondary root is particularly well-developed and clustered. The strong root system enables the absorption of water and nutrients. Several bioactive components have been extracted in acRoots, including polysaccharides, triterpenes, phenols, etc.8-10 Those components have been shown to be anti-cancerous,11 immunoregulatory12 and cardiovascular protective.9
2.2 Chemical composition
2.2.1 Polysaccharides
Actinidia chinensis Planch contains polyphenolic acid. The main active compound is Actinidia chinensis Planch polysaccharide (ACP).13 Traditionally, the hot water extraction method is used to extract ACP, as the diffusion rate of polysaccharide increases in hot environment.14 However, this method consumes a significant amount of energy and is very time-consuming. Therefore, researchers developed other techniques to extract ACP. Han et al. compared the methods of microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE). After analyzing the uronic acid contents, the degree of esterification and molecular weight etc., the MAE method was found to be superior than the UAE method.15 Moreover, the ACP antioxidant activities were evaluated, revealing 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH) and 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate (ABTS) radical-scavenging activities. The ACP antioxidant activities were more pronounced when extracted using the MAE method.15ACP was also shown to have anti-bacterial, anti-viral and anti-tumour effects.16, 17
2.2.2 Triterpenes
Triterpenes is one of the major components of Actinidia chinensis Planch. Wen-Jun Zhu et al. isolated 11 triterpene monomers from Actinidia chinensis Planch. In addition, their study demonstrated that four triterpenes such as asiatic acid exhibited significant antiangiogenic activity in a dose-dependent manner.18 Yixin Xu et al. discovered a new triterpenoid, namely (2α, 3β)-2,3,23-trihydroxyurs-13(18)-en-28-oic acid.19 They also tested the inhibitory effects of six triterpenes including 12α-chloro-2α, 3β, 13β, 23-tetrahydroxyolean-28-oic acid-13-lactone on the cytochrome P450 (CYPs) enzyme activity. Interestingly, CYP3A4 activity was inhibited by up to 90% by 12α-chloro-2α, 3β, 13β, 23-tetrahydroxyolean-28-oic acid-13-lactone.19 Higher CYP3A4 expression levels have been reported in many cancers compared to normal tissues.20 These results indicate that this monomer is worthy of further research as it may have clinical applications. Up to now, about 42 triterpenoids have been isolated and identified from the acRoots. Ursolic acid and oleanolic acid have been identified as the two main anti-tumour components, which regulate apoptosis, ferroptosis and mesenchymal phenotype.21 The effects of these triterpenoids on diseases need to be further studied, especially their anti-cancer abilities.
2.2.3 Phenols
Jun Chang's group isolated twelve phenolic compounds from the acRoots. Among these phenols, planchols A-D were reported for the first time. Planchols A and B demonstrated remarkable anti-proliferation effects on the cytotoxic activity in mouse leukemia 388 and human lung cancer A549 cell lines.22 Tie Zhao et al. then isolated fifty phenol compounds from the root of Actinidia chinensis Planch in 2014. Many of these components were firstly reported, including scopoletin, scoplin, quinic acid derivatives, etc.23
2.2.4 Other compounds
In addition to polysaccharides, triterpenes and phenols, other compounds were also isolated from acRoots, including coumarins,24 flavonoids, lignoceric acid, daucosterol, and so on.1 Similar to the phenolic constituents of acRoots, isomeric flavonoids were also demonstrated to be cytotoxic.22 The volatile oils of acRoots were profiled by Gas Chromatography-Mass Spectrometer, and octane, dodecane, camphor, decane, paeonal, n-decanoic acid, undecane, 4-Methyldodecane and linalool oxide were identified as the principal oils in the roots.1, 25
2.3 Pharmacological functions
2.3.1 Cardiovascular protective activities
Studies generally believed that the consumption of kiwifruits has a preventive effect against obesity and cardiovascular disease.26 Qiang Wang et al. found that the polysaccharide extracted from Actinidia chinensis Planch alleviated Ang II-induced cardiac injury by inhibiting apoptosis. Furthermore, it was reported that the cardioprotective effects may be mediated via the ERK 1/2 and PI3K/Akt signaling pathways.9 Our group also evaluated the role of acRoots in cholesterol metabolism and discovered that acRoots influenced the expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), down titrated low density lipoprotein (LDL) receptor expression and LDL uptake,27 while PCSK9 is well known as one of the main pathogenic genes in familial hypercholesterolemia.28 In recent years, clinical outcome trials showed that PCSK9 inhibitors significantly reduced the production of LDL and the risk of cardiovascular-related diseases.29
2.3.2 Anti-oxidant activity
The antioxidant activity has been shown as one of the most notable function of kiwifruit .30 Liping Qu et al. evaluated the effects of kiwifruit extract on carbon-tetrachloride-(CCl4)-induced acute liver injury. The extract exhibited antioxidant activity by scavenging hydroxyl and DPPH free radicals, relieving liver damage.31 The ethanol-water extract of kiwifruit was fractionated into n-butanol and enriched with oleanolic acid (OLA). Studies also indicated that antioxidant activity might be related to OLA. The OLA-treated liver injury group exhibited greater hepatoprotective activity, with significantly reduction of alanine transaminase and aspartate transaminase levels compared to other extract groups.32 Another monomer of the extract of kiwifruit roots, 2α,3α,24-Thrihydroxyurs-12-en-28-oicacid, showed anti-oxidant activity by triggering cell mitophagy through a reactive oxygen species (ROS)-dependent pathway.33
2.3.3 Cytochrome P450 enzyme inhibitory activities
Yi-Xin Xu et al. evaluated the cell cytochrome P450 (CYPs) enzyme activity of six main triterpenoids, which showed different inhibitory effects on CYPs, especially inhibiting the catalytic activities of CYP3A4.19 Human cytochrome P450 3A (CYP3A) is the largest subfamily of CYP enzymes, which is responsible for metabolic activity.34 Our group also revealed that acRoots regulated some metabolic genes, such as cytochrome P450 family 4 subfamily Z member 1 (CYP4Z1), histidine rich glycoprotein (HRG), matrix metallopeptidase 2 (MMP2), etc., in the treatment of lung cancer (data were not shown). CYP4Z1 is a member of the CYPs family. Reports discovered it had the capability of metabolizing arachidonic acid to 20-hydroxyeicosatetraenoic acid or 14,15-epoxyeicosatrienoic acid and contributed to the cell biology.35 HRG was reported being involved in glucose metabolism.36 MMP2 was reported negatively regulating phospholipase A2 expression and being involved in the cardiac biology, inflammation and lipid metabolic.37 However, more in-depth research is needed to understand better about these metabolism-related genes.
2.3.4 Anti-cancer activities
acRoots is a traditional Chinese medicine that is used for the treatment of various types of cancer.18 The acRoots component ACP, which contains ursolic acid and oleanolic acid, was reported to regulate gastric cancer HGC-27 cell apoptosis, mesenchymal phenotype and ferroptosis.21 The findings indicated that ACP was a promising anti-cancer agent for treating gastric cancer. The extract of acRoots also promoted cell apoptosis and suppressed the cell migration of hypopharyngeal carcinoma cells by regulating the E2F transcription factor 1 (E2F1) and lncRNA MNX1-AS1 pathway.38 Our group also found that acRoots could inhibit cholesterol metabolism and growth of LM3 cells. More research revealed that PCSK9, a secreted serine protease, down titrated LDL receptor expression level and LDL uptake and played a part in vascular events. Cholesterol played an important role in cellular metabolism, particularly for anabolic steps, and influenced the cell growth and division, which were especially relevant to tumour progression.39 Therefore, the researchers found that PCSK9 was also a key gene in regulating hepatocellular carcinoma (HCC) cholesterol metabolism microenvironment with acRoots treatment.27 Fang et al. showed that acRoots (10 mg/ml) could inhibit the proliferation of HCC without cytotoxic effects on the normal cell line L02. The inhibition of the HCC activity was regulated by the distal-less homeobox 2 (DLX2) gene in an epithelial-mesenchymal transition-related manner.40 Furthermore, they found that the DLX2 gene was a transcription factor of the TARBP2 subunit of RISC loading complex (TARBP2) gene, which was associated with poor prognosis in HCC patients. acRoots down-regulated TARBP2 expression in HCC cells.41 These two studies indicated that acRoots suppressed HCC development through the DLX2-TARBP2 pathway. The DLX homeobox family plays indispensable roles in embryonic development and hematological malignancies.42 TARBP2 is involved in miRNA processing and epigenetic biology. It was found to be involved in some cancers.43 The relationship between acRoots-related pathways and cancer immunity or cancer epigenetics requires further investigation (Figure 1).
3 THE ROOT OF ACTINIDIA CHINENSIS PLANCH IN CANCER IMMUNITY
3.1 Cancer immunity
Cancer results from genomic instability and uncontrolled cell growth.44 Cancer progression is driven by cancer cells themselves and their microenvironment.45 The immune system plays a double-edged role in cancer development. Here, we outline some of the immune cells in the tumour microenvironment (Figure 2). For example, CD4+ T cells are involved in the antibody response, and CD8-expressing T cells exert tumour cytotoxic activity,46 while Foxp3+ regulatory T cells (Treg) inhibit autoimmunity and are indicators of poor prognosis in several cancers.47 Tumor-associated neutrophils (TANs) regulate cancer progression, invasion and migration.48 Recent studies showed that N2 neutrophils expressed ROS and suppressed T cell anti-tumour activity. The conversion of neutrophils to the N2 phenotype by transforming growth factor beta 1 (TGF-β1) suggests that TGF-β1 receptor-targeted drugs such as Galunisertib have potential benefits in cancer treatment.49 Similar to TAN, there are abundant tumour-associated macrophages (TAM) in the tumour microenvironment. TAM is classified into two functional subtypes, M1 and M2. M1 exerts anti-tumour functions, whereas M2 promotes cancer progression.50 Despite exerting opposing effects on cancer progression, they also influence each other in the tumour microenvironment. For example, myeloid-derived suppressor cells (MDSCs) were reported being regulating TAM differentiation and affecting tumour progression via the signal transducer and activator of transcription 3 (STAT3) pathways.51 Tregs showed its suppressive effects on the cytotoxic T-cells against tumours by inducing the production of TGF-β.52 Also, MDSCs were reported to be involved in the regulation of Natural Killer (NK) cell functions and they influenced the innate immune response in the tumour microenvironment.53
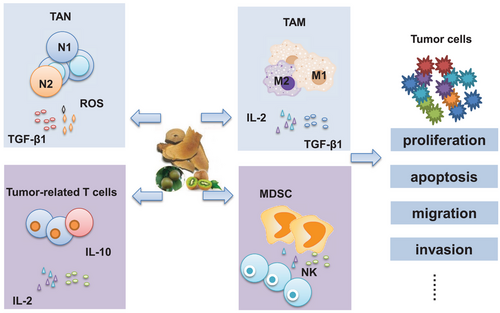
Besides the immune cells, other systems, such as the complement system and inhibitory molecules, also affect cancer immunity. For example, complement effectors such as complement C3a (C3a) and complement C5a (C5a), and their receptors were associated with the inhibition of anti-tumour ability by activating immunosuppression.54 Agents that target cytotoxic immune cells, such as programmed cell death receptor-1 (PD-1) and its ligand, have been extensively studied in cancer immunotherapy.55 Inhibitory molecules such as Tyrosine-protein kinase Mer (MerTK) blockade have also been investigated in recent years to treat cancer. Studies revealed that MerTK inhibitors increased anti-tumour immunity by triggering a type I interferon response.50, 56
3.2 acRoots and cancer immunity
Chinese herbal medicines were reported playing a crucial role in the tumour immune system by regulating different types of immune cells, such as Cytotoxic T cells, NK cells, Tregs and so on.57 acRoots was found to influence a variety of immune cells.
3.2.1 acRoots and T cells
Sun Hong-Xiang et al. explored the effects of kiwifruit roots on the tumour immune system. They found that polysaccharide monomers not only inhibited tumour growth but also significantly influenced the immune cells and immune-related cytokines.58 For example, kiwifruit roots regulated the cytokines of helper T cells Th1 and Th2, such as Interleukin-2 (IL-2) and Interleukin-10 (IL-10), and balanced the Th1/Th2 response in immunized mice.59 Meilin Qu et al. constructed a prognostic model of the extract of acRoots in hepatocellular carcinoma composed of 3 immune-related genes, epidermal growth factor, baculoviral IAP repeat containing 5 (BIRC5), and secreted phosphoprotein 1 (SPP1).60 BIRC5 was highly associated with CD8+T cells and promoted PD-ligand 1 (PD-L1) levels in lung cancer cell A549 but exerted opposite effects in NCI-H1299 cells, which lack p53 expression.61 These results were consistent with our previous study of the acRoots on lung cancer proliferation and gene expression.11 In our result, we found that the NCI-H1299 lung cancer cells were more sensitive to acRoots than other lung cancer cell lines, which highly express p53 gene. It is still unclear that whether the extraction of acRoots influenced tumour immunity through p53 gene regulation. Further studies are required to reveal the underlying mechanism. Triterpenes were reported to play an important role on the immune system. Kim et al. revealed that triterpenes like oleanolic acid inhibited Concanavalin A and induced T cell proliferation in an experimental autoimmune encepholomyelitis model.62 Glycyrrhetinic acid, one of the triterpenes, enhanced a greater Th1 immune response than Th2 response.63 Moreover, Glycyrrhetinic acid promoted lymphocyte proliferation and improved the proportions of T cells when encapsulated with liposomes.64 Phenolic also showed its pro-inflammatory or anti-inflammatory effect through various pathways in previous studies.65 However, except polysaccharide, other acRoots extracted monomers in tumour immunity are rarely studied. The extraction methods need to be improved to increase the monomer's amount to do corresponding research.
3.2.2 acRoots and macrophages
In the previous research, Traditional Chinese Medicine with low-dose sorafenib induced the M1 status and triggered the TNFR1-MAPK signaling pathway in the HCC microenvironment.66 Polysaccharide was reported as an important component of acRoots extract and could degrade suppressor of cytokine signaling (SOCS1/2) and promote M1 polarization.67 Similar studies also revealed that polysaccharides enhanced the production of NO, the expression of IL-6 and iNOS through the NF-κB p65/MAPK signaling pathway.68 An in vivo study further uncovered that polysaccharides might act as a toll-like receptor 4 (TLR4) agonist and promote immune responses.69 Sun et al. also found that the roots of kiwifruit enhanced the phagocytic function of macrophages and influenced the inflammatory-related genes of RAW264.7 cells through the NF-κB and microRNA pathways.70, 71 Further research showed that combination treatment with kiwifruit roots and anti-PD1 decreased N-cadherin, KLF transcription factor 4 (KLF4) and POU class 5 homeobox 1 pseudogene 4 (Oct4) expressions and increased interferon gamma (IFN-γ) and TNF-α expression. The combination treatment prolonged the survival of colorectal cancer-xenograft mice.72 This indicated that kiwifruit roots might be a potential candidate in cancer adjuvant therapy. The monomers of acRoots on macrophages effect were rarely studied. Triterpenes were also reported to influence the iNOS system and affect the macrophages’ differentiation.73 Similarly, phenolic composition like apigenin was reported to modulate microglial activition by inducing a STAT1-CD40 pathway.65 Likewise, the role of acRoots’ monomers in macrophages needs to be further researched.
3.2.3 acRoots and potential immune-related genes
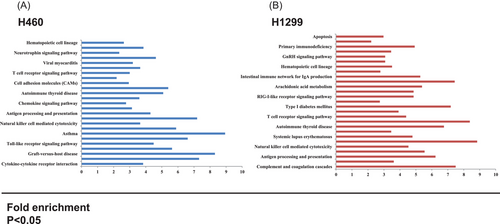
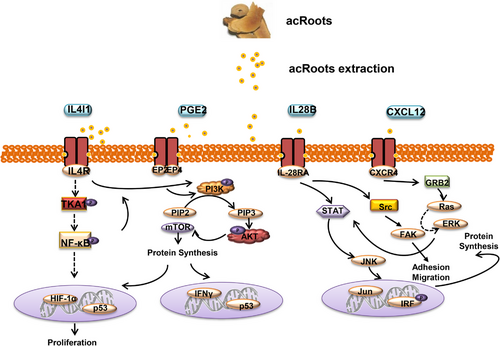
Besides the T cells and macrophages, the kiwifruit root extract could also increase NK cell activity.59 However, in-depth research on the effects of acRoots on immune cells in the tumour microenvironment is still lacking. Our group investigated the effects of acRoots on lung cancer cells with or without p53 gene expression, revealing 2137 regulated genes in p53-expressing lung cancer cells and 2137 regulated genes in lung cancer cells without p53 gene expression. These genes were enriched in the neurotrophin signaling pathway, T cell receptor signaling pathway, Toll-like receptor signaling pathway, complement and coagulation cascades and so on (Figure 3). These genes were analyzed, and the top 12 up-regulated and down-regulated genes were selected for further analysis, including 2′-5′ oligoadenylate synthetase-like (OASL), prostaglandin E synthase (PTGES), interleukin 28B (IL-28B), TNF, C-X-C motif chemokine receptor 4 (CXCR4) and so on. The OASL gene is a member of the OAS family. Research showed that OASL played an immune-related role in cancers and viral infections. OASL−/− mice demonstrated increased IFN production and enhanced the cytotoxic function of CD8+ T cells and NK cells.74, 75 In addition, the PGE2/PTGES pathway plays a key role in inflammation.76 Studies revealed that PTGES/PGE2 signaling protected tumour cells against T- cell cytotoxicity and recruited MDSCs to the tumour microenvironment.77 IL-28B, TNF and CXCR4 are cytokines and chemokines related to the immune system. IL-28B was found to inhibit HCV replication by inducing the phosphorylation of STAT1 and STAT2.78 TNF and its inhibitor are now used in the treatment of inflammatory diseases.79 TNF was shown to induce endothelial cells to express cyclooxygenase 2 (COX2) and release prostaglandin.80 CXCL12/CXCR4 signaling controls multiple components of immune cell development, such as T cells, MDSC cells, and NK cells. CXCL12/CXCR4 signaling promoted migration of MDSCs and Treg cells into the tumour microenvironment and therefore impaired the anti-tumour response.81, 82 The anti-tumour effects of OASL-PI3K signaling of the acRoots on lung cancer were investigated.83 In our another acRoots treated HCC cell lines, we also found some immune-related pathways were enriched when treated with the extraction of acRoots, such as Inflammatory bowel disease pathway, NK cell mediated cytotoxicity pathway, TGF-β signaling pathway and so on. Similarly, immune-related genes like interleukin 4 induced 1, interferon alpha 21 and C-X-C motif chemokine ligand 2 were significantly changed when treated with acRoots. However, the role of these genes found in acRoots in the treatment of tumours requires further study (Figure 4).
4 CONCLUSIONS
acRoots have been used in the treatment of cancer and cardiovascular diseases. acRoots contain many monomers such as polysaccharides, triterpenes, phenols and so on. The monomers of the acRoots exhibit anti-viral, anti-proliferation and anti-tumour effects. Polysaccharides are a widely studied monomer of acRoots and vast researches focused on the anti-cancer ability. However, other monomers are less studied due to the extraction methods and limited amounts. Our unrevealed study indicated coumarin extracted from acRoots has anti-cancer effect. More in-depth researches are required to further investigate the efficacy of monomers in cancer immunity. With the development of omics, the mechanisms of action of acRoots have been revealed. The immune-related genes such as PTGES, CXCR4, TNF etc. were revealed to have the potential role in acRoots treated tumour immune microenvironment and influence the tumour progression. Since the extracts contained multiple components, the mechanisms of actions might not be accurately identified due to mutual interference. Nevertheless, such research effectively isolates monomers for subsequent studies. Further in vivo studies should also be performed to investigate potential treatment options.
ACKNOWLEDGEMENTS
The work was supported by Operation Funding of Shanghai Institute of Clinical Bioinformatics and Shanghai Engineering and Technology Center for Artificial Intelligence of Lung and Heart Diseases from Zhongshan Hospital, National Natural Science Foundation of China (grant number: 81873409), cross key project of mathematics and medical health of National Natural Science Foundation of China (grant number: 12026608) and by grants from the Shanghai Science and Technology Innovation (grant number: 20S11905000 to XZ) and the Clinical Research Plan of SHCD.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The paper was handled by editors and has undergone a rigorous peer-review process.




