Gut microbiota intervention strategies using active components from medicinal herbs to evaluate clinical efficacy of type 2 diabetes – A review
Abstract
There is a myriad of phytocomponents in various medicinal herbs, and some of these substances have low absolute oral bioavailability. There is a complex and nuanced interaction between metabolic profiles and gut microbiota that influences human health and illness. An important component of alternative and complementary health care is the use of medicinal herbs for therapeutic purposes. Expanding facets from numerous scientific discoveries mentioned the potential linkage between intestinal microbiota and the curative capabilities of beneficial components from medicinal herbs upon their recognition. There is a strong interaction between gut microbiota and host at the mucosal barrier of the gastrointestinal tract. This reinforces the notion that it is important to consider the effects of medicinal herbs in relation to gut flora and metabolic disorders, including type 2 diabetes. There has been an explosion in the number of medicinal herbs coming into the spotlight with phytocomponents already recognised as having anti-diabetic effects, such as increased insulin sensitivity and decreased blood sugar levels. The review sites' assertion that altering gastrointestinal microbial community by intervention tactics that modulate the gut microbiota by using phytocomponents from medicinal herbs with a major emphasis only on flavonoids, alkaloids, glycosides, and terpenoids may very well be relevant to T2DM rehabilitation may indeed be relevant. The goal of this review is to present an overview of the potential impacts of using medicinal herbs for the prevention and treatment of type 2 diabetes by modifying the gut microbiota in a healthier manner. Based on the available scientific literature, in order to gain a deeper understanding of how to integrate specialised treatments that are based on intestinal bacteria into mainstream clinical practice, this review is aimed at providing a comprehensive understanding of the pathogenesis and progression of T2DM, as well as more thoughtful strategies to manipulate the gut microbiota with medicinal herbs as an integral part of intervention strategies.
1 INTRODUCTION
1.1 Type 2 diabetes mellitus (T2DM), intestinal microbiota and medicinal herbs
1.1.1 Type 2 diabetes mellitus (T2DM)
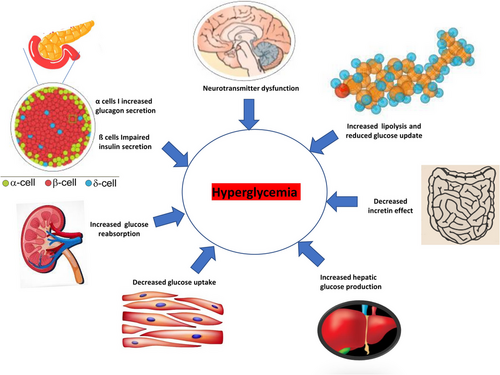
T2DM is caused by a complex chain of events involving genetic, epigenetic and environmental factors.1 The development of T2D is usually preceded by insulin resistance, which impairs insulin's effectiveness on peripheral tissues such as peripheral muscles, livers and adipose tissues.2 As a result of a persistently elevated blood glucose level (hyperglycaemia), T2DM causes abnormalities of the beta cells, insulin resistance, glycolipid dysfunction, and abnormalities of the islet cells.3 The abnormalities in other hormones, such as decreased incretin glucagon-like peptide 1 (GLP-1) production, hyperglucagonaemia and increased levels of other counter-regulatory hormones, are the root causes of T2DM.4-7
A prolonged hyperglycaemia can result in oxidative stress and inflammation, which are common symptoms of type 2 diabetes.3, 8-10 A further consequence of the rapid development of society and the economy is a change in traditional lifestyles, behaviour patterns, and lifestyle choices, which then lead to an increase in type 2 diabetes prevalence.11
A variety of undesirable health habits have been responsible for the worldwide increase in the incidence of T2DM, the most prominent being inactivity, poor nutrition, smoking, and excessive alcohol consumption.12, 13 The mortality rate among adult patients with T2DM is two to four times greater than that of those without diabetes, according to several studies.14-20 The overall T2DM manifestation associated to hyperglycaemia has been described in Figure 1.
1.1.2 Gut microbiota
Recent years have witnessed a gradual awakening among people that functioning of our metabolism is determined by more than ourselves; it is closely linked to microbiota of our gut, that is termed as ‘second genome’.22 It is apparent that there is a considerable heterogeneity in both abundance and identification of microbes (bacteria, archaea, fungi and viruses) in the human gut, which suggests that these microbes have a multifaceted habitat throughout the gastrointestinal system.23 The healthy gastrointestinal tract is dominated by Bacteroides, Firmicutes and Actinobacteria with relatively few Proteobacteria, Verrucomicrobia and other microbial species.24-26
A healthy intestinal microbes is developed as a result of a plethora of variables including nutrition, environmental conditions, genetics and antibiotics.27 Every person possesses one-of-a-kind profile of the gut microbiota, which is responsible for a wide variety of functions in the body of the host.28 The colonisation of intestinal flora functions in a manner that is analogous to the development of the immune system.29 It also has a big effect on how the digestive system works and controls the metabolic pathways.30
The interplay between the microorganisms present in the gastrointestinal tract and the host is always dynamic and, for the most part, harmonious in every healthy individual.31 It is a key part of immunity32, 33 keeping overall health in a good shape.34 In summary, the intestinal microbes can act as a barrier to keep some pathogens away from getting into organs and tissues.35 It has been proposed that an imbalance in the intestinal microbial community is actually associated with the emergence and advancement of various metabolic illnesses including diabetes.36, 37 The microbes inherited into gastrointestinal tract are capable of generating a variety of secondary metabolites, such as amino acids and various kinds of fatty acids (SCFA). The preponderance of these secondary metabolites has had the capacity to interact with specific receptors and actually participate in the maintenance of the host's usual physiological mechanisms under ideal conditions.38-41
1.1.3 Medicinal herbs and phytocomponents
One category of medicines that are derived from plant source is referred to as ‘medicinal herbs’. These medicines might also go by the names of botanical medicines or phytomedicines.42 Medicinal herbs can be herbal substances that have been refined, herbal extracts in their raw form, herbal concoctions and so on.43 In certain circumstances, the idea of medicinal herbs can be expanded to include certain compounds that derived from fungi, minerals, or even animals (World Health Organization, 2000).44 Alkaloids, flavonoids, terpenoids and glycosides are among the various phytochemicals observed in medicinal herbs. In vivo and in vitro studies carried out by numerous research groups have well speculated that all these compounds may have prospective biological activity that functions as a modulator of gut microbiota.10, 45
Due to the growing prevalence of antimicrobial resistance to some antibiotics, the use of phytochemicals to mitigate the potentially harmful effects of intestinal bacteria in some diseases is becoming increasingly common now on a daily basis.46, 47 It has always been demonstrated that low levels of active ingredients that come from medicinal herbs or other plant compounds have always had the ability to inhibit the growth of pathogenic microbiota in the gastrointestinal system.48 Aside from controlling signal translation or genetic expression with phospholipoid cell membranes, the various phytochemical compounds from medicinal herbs can significantly reduce the growth of disease-causing bacteria in the gut. Furthermore, they can interfere with microbial metabolic functions, improve barrier properties, cause cell component damage and disrupt the balance of enzymes involved in the production of cellular energy and the synthesis of cellular substances.49-53
Traditionally, the overwhelming majority of medicinal herbs that utilised in the management of diabetic comorbidities significantly alter the metabolic functions in effective way.54 This would include gluconeogenesis, glycolysis, oxidative phosphorylation, glycogen synthesis and breakdown, production as well as insulin release, cholesterol metabolism, glucose metabolism and its absorption.55, 56 However, the microflora in the gut is progressively being investigated as a potential therapeutic target for diabetes. Given the favourable performance of active agents form the medicinal herbs, particularly in the regulation of gut microbiota, there should be more consideration given to the utilisation of medicinal herbs in the treatment of diabetes.57
1.2 Mechanism of interplay involving T2DM, gut microbiota and phytocomponents from medicinal herbs
To develop novel intestinal approaches to manage T2DM and/or its complications, we present the relationship between T2DM, components from medicinal herbs, and the intestinal microflora in this review. The microbiota are primarily responsible for sustaining endocrine and neurological communications in the intestine, in addition to the host's immune system.58 Moreover, they also control the medication activity including metabolic activity, remove impurities, and create different components that exert an influence on the host.59
In the digestive tract, microbes uptake active components from medicinal herbs and transform them into absorbable, functional molecules through a variety of metabolic pathways.60 These molecules subsequently enter the body and exhibit functional pharmacological action. Likewise, medicinal herbs components have the ability to regulate the composition of gut microbiota and its secretions.61 If somehow the constitution of intestinal microbiota as well as its secretions is altered, then perhaps this modification in turn does have a therapeutic benefit.62
In light of the currently available information on T2DM and intestinal microbiota, it has been hypothesised that patients with T2DM have a more distinct gut microbial composition than healthy individuals. However, the specific gut microbiota markers of diabetic problems have not been identified yet.63 In recent studies, the alterations in the gut microbiota associated with diabetic comorbidities are generally characterised by reductions in favourable microbes, such as butyrate-producing bacteria and microbes associated with bile acid (BAs) metabolism, and elevations in conditional pathogenic bacteria. Even though these gut microbes' attributes are not exclusive to T2DM and associated disorders, they are prominent in other illnesses as well, notably Parkinson's disease and colon cancer.64-66
Intestinal flora is found to be a significant factor in controlling metabolic activity, appetite, tolerance, glucose homeostasis and excess body weight in humans.67-70 The altered composition of microbial communities has been attributed to T2DM as a metabolic condition.9, 71 Modifications in the intestinal microbiota have been attributed to the pathophysiology of insulin sensitivity, and several strategies that assist in elucidating such a relationship have been documented.72 Of the most prevalent discoveries, the genera Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were significantly negatively correlated with T2DM. On the other hand, the genera Ruminococcus, Fusobacterium and Blautia were shown to have a positive correlation with T2DM.73 Both the ratios, e.g., Bacteroidetes to Firmicutes and Bacteroides-Prevotella group to the C. coccoides-E. rectale group, were primarily linked to levels of glucose. In addition, representatives of the Betaproteobacteria appeared substantially abundant in T2DM patients.37 In addition, a case-control research revealed that individuals who have T2DM and obesity have less Lactobacillus versus controls, specifically the subgroups L. acidophilus, L. plantarum and L. reuteri.74 There is some evidence from a number of studies suggesting that the ratio of Firmicutes to Bacteroidetes is higher in patients with diabetes than in healthy people.75, 76
1.3 Role of gut microbiota in the pathophysiology of T2DM
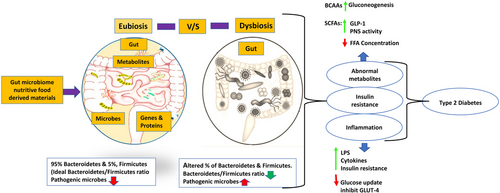
In general, aging is associated with a decrease in the diversity of gut microbes. Several factors can affect the gut microbiome of a developing person, including lifestyle choices, physiological variations, eating practices, nutritional deficiencies and several medications, including antibiotics and prescribed drugs.77, 78 An accumulating body of research has found that dysbiosis, an imbalance in the gut's microbiota caused by a reduced diversity and widespread prevalence of certain pathogenic taxa, has been linked to a wide range of human diseases.79-81 Several diseases have been linked to dysbiosis, such as inflammatory bowel disease and colorectal cancer82, 83 as well as obesity, diabetes, asthma and allergies84, 85 and its progression is due to a number of factors that influence gut dysbiosis. A gut dysbiosis is characterised by a decrease in bacterial diversity, loss of beneficial microorganisms, or an increase in potentially dangerous microorganisms, according to available literature.86, 87
1.4 One of the potential mechanism of gut dysbiosis and T2DM
Recent research suggests the following factors may play a crucial role in this overall system during intestinal dysbiosis impacting glucose metabolism: (1) LPS (lipopolysaccharides): LPS is a critical component of the outer membrane of Gram-negative bacteria, and excessive LPS in the bloodstream can produce low-grade inflammation linked with diabetes.88 (2) SCFAs (short-chain fatty acids): SCFAs, which often constitute acetic acid, propionic acid, and butyric acid, are indeed the principal fermentation products created from indigestible fibres and polysaccharides, and diminished SCFA contents may impair the host's metabolic equilibrium since they are connected to energy metabolism, GLP-1 secretion, and the integrity of the intestinal mucosa.89 (3) BA (bile acids): Produced by the breakdown of cholesterol, BAs are metabolised by the gut microbiota. Any disturbance of the intestinal microbiota constituents may affect intestinal tight junction proteins, increases the gut permeability, causes the persistent LPS leakage followed by systemic low-grade inflammation.90 (4) Energy harvest: a gut microbiota that is associated with obesity is more effective at utilising energy from the diet, which results in an energy overload in the host and is a fundamental foundation for the formation of insulin resistance and T2DM.91
In patients withT2DM, dysbiosis is the root cause of insulin resistance, both at the onset and throughout the disease.92 An unbalanced composition of the microbiota in the gut might also lead to the accumulation of toxic components produced by bacteria. According to microbial phylogeny, an abnormality in the microflora of the gut and an increase in the permeability of the intestine are the two elements that lead to the development of obesity before diabetes develops.93 Flagellin, lipopolysaccharide, peptidoglycan and lipoteichoic acid are just a few examples of the molecular patterns associated with disease-causing microorganisms that affect both the normal functioning and occurrence of disease in the host body. This is controlled by receptors that have the capacity to recognise patterns, such as toll-like receptors. These receptors respond to tissue damage and the presence of pathogens in two different ways. As a result, cytokines are produced.94 These toll-like receptors have the capability of recognising ligand molecules, including endotoxin, which are produced by cells of the immune system and possess the potential to trigger inflammation. In instances of metabolic disorders including diabetes as well as obesity, there has been an increase in both the number of toll-like receptors as well as the activity of these receptors.95, 96 Patients with T2DM have increased levels of inflammatory cytokines in their serum, including interleukin-6, interleukin-18, and tumour necrosis factor alpha.97, 98 Also, the lipopolysaccharide layer of Gram-negative bacteria has been linked to how diabetes mellitus develops.99, 100
Intestinal dysbiosis modifies the microbiota by increasing sugar transfer across membranes and branched-chain amino acid transport while decreasing butyrate production.101 A direct link between the altered microbiota composition and the inflammatory state in patients with T2DM is suggested by the enhanced microbial genes that were linked to the oxidative stress signalling pathway.102 When the gut barrier is compromised due to dysbiosis, it causes inflammation both locally and throughout the body, which may play a significant role in the onset of type 2 diabetes.103 The mechanism of action on involvement of gut dysbiosis on T2DM pathophysiology has been depicted in Figure 2.
1.5 Role of microbiota in T2DM treatment
The development of T2DM is influenced by the intestinal microbiota and its metabolites, and the process can be partially reversed by controlling the intestinal microbiota and its metabolites, offering a unique perspective to clinical treatment.106
1.6 Diet and physical activity
Patients who haveT2DM should place extra emphasis on optimising their carbohydrate consumption and increasing the amount of dietary fibres taken daily. It is already well postulated that dietary intake of fibres plays a significant part in both the process of controlling metabolism as well as warding off persistent gastroenterological conditions.107 Microbial fermentation of dietary fibres leads to the formation of short-chain fatty acids (SCFAs), which have been shown to reduce levels of cholesterol and manage glucose levels.108 Enhancing physical activity and fitness is another essential aspect of relieving T2DM. Blood sugar levels can be lowered and insulin sensitivity can be increased with regular exercise.109 It has also been demonstrated that regular physical activity can improve the make-up of the gut microbiome, boost the formation of short-chain fatty acids (SCFAs), and lower plasma concentrations of SCFAs, which can help reduce insulin resistance in skeletal muscle.110 Exercise-induced improvements in SCFA-producing microbes and intestinal barrier integrity are also significant considerations in type 2 diabetes improvement.111 Importantly, the abundance of Akkermansia muciniphila and the cumulative diversity of the athletes’ microbial community have both been shown to significantly increase with exercise.112
1.7 Prebiotics, probiotics and synbiotics
The clinical application of prebiotics, probiotics and synbiotics for strengthening glycaemic control in patients diagnosed with T2DM is endorsed by a growing body of literature.113, 114 Increased regulation of T2DM has been associated with the use of probiotics, which have been shown to modify the composition of the gut microbiota in a way that benefits the body overall. This includes increased intestinal integrity, significantly reduced circulating LPS, lowered endoplasmic reticulum stress, and augmented peripheral insulin sensitivity.115 The effects of probiotic supplementation on HbA1c levels, fasting blood glucose (FBG) and insulin resistance in T2DM patients were the focus of a meta-analysis conducted by Tao et al. Fifteen randomised controlled trials (RCTs) involving 902 patients were included. The findings suggested that probiotics have the potential to lower HbA1c levels (p = .02), fasting blood glucose levels (p = .003) and insulin resistance (p < .00001) compared to the control group.116 The most comprehensive meta-analysis to date was published by Wang et al.117 They glanced at 33 randomised controlled trials (RCTs) with a total of 1346 participants from healthy, obese and T2DM cohorts. When comparing the prebiotics supplemented groups to the control group, those with prediabetes or type 2 diabetes saw reductions in fasting blood glucose, hemoglobin A1c, fasting insulin concentration, and insulin sensitivity of 7.15%, 7.00%, 16.58% and 25.34 %, respectively.117 For consistent improvement across all glycaemic indicators, it was suggested that a daily supplement dose of more than 10 g be taken for a minimum of 42 days.117 Sixty T2DM pre-hypertensive patients were given a synbiotic by Sheth et al.118 (two species of Lactobacillus and Bifidobacterium each, one species of Streptococcus and yeast, and 300 mg oligosaccharide). As a result of the intervention, participants saw decreases in enteric pathogens (44.6%), increases in fasting blood glucose (3.9%), and decreases in HbA1c (14%).118
1.8 Faecal microbiota transplant in the treatment of T2DM
While there is currently scant evidence supporting the use of FMT in T2DM, interest can be piqued by human studies examining the clinical effects of FMT in patients with metabolic syndrome. Several active studies are exploring into other possible indications, such as type 2 diabetes. It is presumed that FMT has greater potential than dietary supplements like probiotics due to its ability to transfer complete donor microbiota communities along with their metabolites and its presumed superiority in repairing microbiota disruption over single microbial targets.119 Changes in the intestinal microbiota composition have been linked to improvements in metabolic syndrome patients' HbA1c levels following allogenic FMT, as was reported in a study performed by Koottey et al.120 Six weeks after receiving allogenic microbiota, Vrieze et al.121 discovered that male recipients with metabolic syndrome had improved insulin sensitivity and a greater abundance of butyrate-producing intestinal microbiota (the species Roseburia intestinalis).
1.9 Medicinal herbs in the management of type 2 diabetes
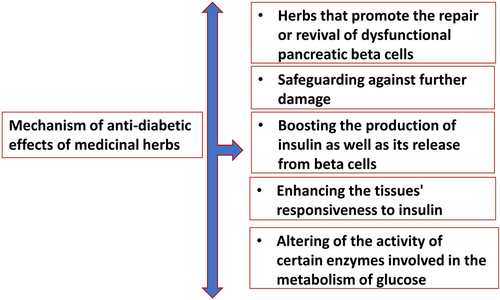
Many medicinal plant phytocomponents were evaluated for their hypoglycaemic effects and were found to be effective in both animal models and human clinical trials. The mechanism of the anti-diabetic benefits of various medicinal herbs has been elucidated, and their bioactive components have been extracted and identified.122 Many other pathways exist by which the medicinal herbs could have provoked hypoglycaemia. Among these were the acceleration of the regeneration or rejuvenation of impaired pancreatic beta cells, as well as the prevention of additional damage, boosting the beta cells' production and secretion of insulin, lowering the amount of glucose that is absorbed by the gastrointestinal tract, increasing tissue insulin sensitivity, exhibiting characteristics similar to those of insulin, in addition to altering the activity of some enzymes involved in the metabolism of glucose123 as shown in Figure 3.
1.10 Medicinal herbs, gut microbiota and T2DM
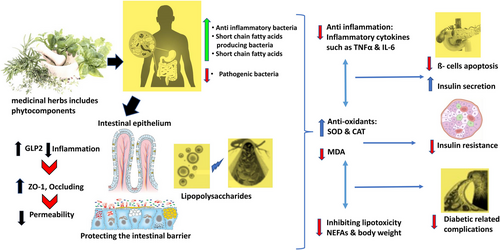
It has been reported that a wide range of herbs and herbal compounds have therapeutic effects on type 2 diabetes by altering the composition of the intestinal microbiota. Active components from the medicinal herbs have anti-inflammatory and antioxidant properties, which can benefit T2DM.124 These characteristics not only help in reviving the islets and lowering insulin resistance, but they also protect against the onset of diabetes-related complications. Elements derived from medicinal herbs are used as prebiotics to combat intestinal microbial dysbiosis.125 This could be a potential therapeutic approach for type 2 diabetes as well as the metabolic changes that are associated with it.126 In conclusion, medicinal herbs have a significant and widespread impact on the gut microbiota.127, 128
1.11 Multidisciplinary intervention strategy for the gut microbiota using active components from medicinal herbs in the treatment of T2DM
The use of prebiotics, probiotics, various medicines like metformin and intervention tactics derived from the contents of medicinal herbs to alter the composition of gut microbiota is an innovative method for managing blood sugar in type 2 diabetes.37 In point of fact, diabetics have been shown to have proposed modifications to the metabolic pathways129 in their bodies prior to the manifestation of various histopathological changes. As a consequence of all of this, the accompanying difference metabolites may have unintended effects as biomarkers for the beginning and advancement of diabetes.130
In addition to this, the microbiota in the gut enhances the activity of pharmacological components that are present in active medicinal herbs; it reduces the cytotoxic action of several of the components that are found in medicinal plants.131 This appears to be an appealing and noteworthy framework for domineering T2DM, as medicinal herbs can indeed mitigate diabetes by a variety of processes, such as gut barrier activity, antimicrobial, anti-inflammatory, gastrointestinal hormone-related diabetes control and insulin release etc., and this framework can be used to control T2DM.132
Human, animal and in vitro studies have indeed investigated further the relationship between both the gut microbiota and medicinal herbs as they pertain to T2DM management. Phytocomponents of medicinal herbs have the ability to regulate the composition of bacteria in the gut, both beneficial and harmful bacteria. In addition, these phytocomponents reduce inflammation that is caused when the gut microbiota is in a dysbiotic state.133 In addition, the metabolic activity of the microbiota in the gut can modulate the biotransformation of phytocomponents from medicinal herbs to better understand the pathogenesis of T2DM, potential role of intestinal microbiota, and the promising therapeutic mechanisms of medicinal herbs phytocomponents in T2DM, we have compiled a summary of such research findings in the current review. This should lead to new insights into targeted medicinal herbs-based therapies with a focus on the gut microbiota.
This review sheds light on potential new avenues for the development of effective preventative measures and therapeutic techniques aimed at curcumin (flavonoids), berberine (alkaloids), terpenoids and ginsenosides (glycosides), which are important phytocomponents from medicinal herbs that have been shown to have ameliorative effects on the intestinal microbiota and type 2 diabetes (T2DM).
1.12 Gut microbiota intervention through flavonoids in treatment of T2DM
1.12.1 Flavonoids
In terms of potential therapeutic activities in a variety of metabolic illnesses, including T2DM, flavonoids are the most important bioactive species in medicinal herbs.134 Despite the fact that several studies have proven that flavonoids from medicinal herbs have a positive effect on the microbiota of the gastrointestinal tract, the exact mechanism by which they produce this effect is still poorly understood.135-137
Flavonoids and gut bacteria have a two-way influence upon one another. Flavonoids can modify gut microbiota in vivo, and microbiome can influence flavonoid absorption and metabolism. Flavonoids have the ability to regulate gut microbiota, which can increase the number of species of beneficial bacteria as well as their diversity. Flavonoids also have the ability to reduce the number of harmful bacteria. It can improve the barrier function of the gut and control both innate and adaptive immune responses, which ultimately results in a reduction in insulin resistance and inflammation. Additionally, the gut flora can alter flavonoids and increase their bioavailability. The combination of flavonoids and gut microbiota can lower the incidence of T2DM and improve comorbidities associated with T2DM138 as shown in Figure 5.
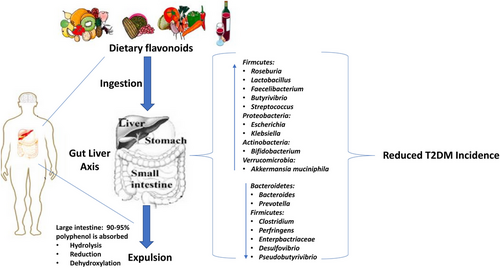
1.13 Curcumin distorts intestinal microflora: a new paradigm for comprehending effects of curcumin on T2DM patients
Both the rhizome and the tuber of the Curcuma plant are sources of curcumin. That compound is a naturally occurring polyphenol. It is no secret that the pharmacological benefits of flavonoids (polyphenols) have been drawing increasing interest around the world in recent years.139-142 These pharmacological effects focus mostly on reducing inflammation and boosting the immune system. Curcumin has a wide variety of health benefits, including potential uses in the treatment and prevention of numerous diseases due to its antibacterial, antiviral, anticancer and anti-diabetic properties.143-145
The anti-inflammatory effects of curcumin were demonstrated by a reduction in the levels of inflammatory mediators in the serum, including IL-6, TNF-α and MCP-1. In addition to this effect, the NF-B signalling pathway was inhibited, which resulted in the cessation of the inflammatory response.146, 147
The effect of curcumin on the intestinal flora is still poorly known. However, the available literature suggests that curcumin gets bio-transformed into such a multiplicity of metabolites not only in the stomach, but also via a range of other routes.148 In addition, curcumin treatment may affect bacteria in the digestive tract, leading to shifts in the diversity and composition of gut microbes.149 This means that in any pathogenic situation, there is the potential for substantial indirect health benefits.148 Curcumin and gut microbes engage in a dynamic, bidirectional interaction, as depicted in Figure 6.148, 150-152
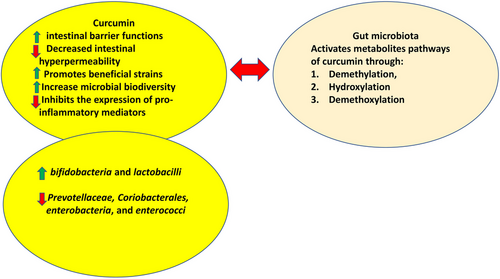
Though curcumin has a poor bioavailability in the body, it is thought that it may have direct regulatory effects on the gut flora.153 This may shed light on a wide variety of observed pharmacological effects.154 By increasing the abundance of butyrate-producing microorganisms like bifidobacteria and lactobacilli while greatly decreasing the loads of Prevotellaceae, Coriobacterales, enterobacteria and enterococci, curcumin supplementation dramatically altered the ratio of beneficial microbes to pathogenic microbes in the gut,155 also showed a prebiotic-like efficacy against gut-based pathogenic microbes.155
In a double-blind, randomised and placebo-controlled pilot research, the human intestinal microbiota showed a highly personalised response to curcumin treatment, with clear responders and non-responders.150 The microbes Clostridium spp., Bacteroides spp., Citrobacter spp., Cronobacter spp., Enterobacter spp., Enterococcus spp., Klebsiella spp., Parabacteroides spp. and Pseudomonas spp. all grew uniformly in these responding participants. The presence and abundance of numerous Blautia species and the preponderance of Ruminococcus spp. diminished, which was typical for these individuals.156
Curcumin ameliorated the HFD-induced dysbiosis of the gut microbiota in diabetic rats. Curcumin has been shown to preserve the intestinal mucosal barrier, enhance insulin resistance and reduce blood glucose levels in diabetic rats. This research has the potential to contribute experimental evidence towards T2DM prevention and therapy.157 Furthermore, Yuan et al. discovered that curcumin was able to reverse dysbiosis in the intestinal microbiota,158 when each piece of documentation on the implications of curcumin is recognised, which includes how it responds well with intervention strategy on intestinal microbiota; it becomes more and more clear that curcumin can protect the individual from the detrimental consequences of diabetes through potential role by modulating the beneficial intestinal flora.
1.14 Alkloids
The potential health benefits of alkaloids, including their effects against germs, cancer, malaria, asthma and hyperglycaemia, are widely recognised. There are several that have been used in both traditional and contemporary medicine to find new medical uses for them.159, 160
1.15 Anti-diabetic abilities of berberine: credited to its capacity to modulate the gut flora
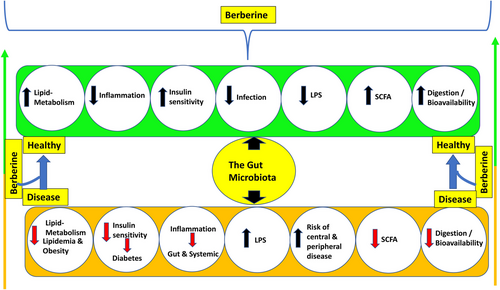
Rhizoma coptidis, often known as berberine, is a pharmacological component found in high concentrations in this medicinal herb. In clinical trials, it improved glucose control and lowered insulin resistance, suggesting a role in diabetes treatment.161, 162 The important consideration was highlighted by several findings that berberine reduces blood glucose levels and boosts insulin secretion, lowering body weight and lipid levels in general. Furthermore, berberine can minimise blood glucose and insulin resistance in experimental studies through stimulating the AMPK pathway, intensifying glucagon-like peptide-1 (GLP-1) thresholds, dampening ROS production, countering mitochondrial dysfunction, diminishing endothelial microparticles-mediated oxidative stress and suppressing inflammation.163-167 Given the premise that berberine's multifaceted impact is progressively associated with the gut flora and showed beneficial impact in several metabloic disorders. According to a growing body of research, berberine is hypothesised to restore pathologically induced structural and numerical modifications in the gut microbiota.168
In a healthy person, berberine's ability to alter the composition of gut flora stands out as the single most fundamental response to this chance. Prior studies169, 170 have proven how berberine seems to have the capacity to annihilate detrimental gut microbes by triggering cell death while at the same time boosting the numbers of beneficial gut microbes like Bifidobacterium adolescentis and Lactobacillus acidophilus.
The clinical trial in diabetes patients was performed by Chen et al.,171 and it showed that the regulation of Bifidobacterium species may be related to the anti-diabetic benefits of berberine in diabetic individuals, which were achieved by giving them 300 mg of berberine three times per day, 0.5 h after each meal, for 8 weeks. When compared to before treatment, the abundance of Bifidobacterium, Bifidobacterium longum, Bifidobacterium breve and Bifidobacterium adolescentis was considerably higher, while the abundance of Bifidobacterium, B. longum and B. infantis dramatically decreased. Bifidobacterium, B. longum and B. infantis also showed significant negative relationships with TNF-a and LPS levels.171 So, berberine's route of action in affecting metabolic disorders appears to be similar to that of probiotics.172-175 Another decisive element to consider is that it suppresses levels of LPS in the gut and prevents the proliferation of Escherichia coli, which would alleviate chronic systemic inflammation.176
As evidenced by their work, Shan et al.177 conclude that berberine can aid in the rehabilitation of both the immune system and the damaged intestinal mucosa, protecting the host's metabolic balance and preventing the entry of intestinal endotoxins into the bloodstream.177, 178 Intestinal bacteria have the capability to break down and metabolise berberine to produce dihydro-berberine, which blocks the intestinal tract's absorption of disaccharides,179 strengthen the intestinal permeability by hindering the TLR4/MyD88/NF-kB signalling pathway178 as well as increases the exudation of GLP-2.180 Medicinal herbs have the ability to ameliorate pathological condition by limiting the number of bacterial pathogens, boosting the number of mucosal preventative microbes and facilitating the production of SCFAs.127, 181
In the contemporary field of medicine, it is universally well recognised that chronic subclinical inflammation frequently accompanies diabetes, and numerous studies182, 183 have demonstrated the importance of inflammation in the aetiology of T2DM and its vascular consequences. In vitro and in vivo studies of berberine's anti-inflammatory properties showed that it may have exerted its hypoglycaemic benefits through reducing inflammation.182 The overall mechanism is been described in Figure 7.168, 178, 180, 181
1.16 Terpenoids and its impact on T2DM-related gut microbiota regulation
Based on the current body of research, it is apparent that some of the medicinal herbs employed in the preparation of herbal formulae can treat a wide variety of disorders, including T2DM.184 Terpenoids are a class of organic compounds with a wide range of structures and roles. They are composed of many isoprene units.185 In general and especially, terpenoids from the medicinal herbs can indeed be best suited for use in pharmacological configurations.186
Terpenoids unearthed in medicinal herbs or dietary plants, that possess the potential to alleviate metabolic disorders through the authentication of ligand-dependent transcriptional regulators, specifically peroxisome proliferator-activated receptors (PPARs).186 However, the exact strategy could be accomplished by safeguarding islet cells and strengthening glucose homeostasis and liver glycogen biosynthesis.187 There are, however, scant data on the detailed action on how terpenoids influence T2DM patients primarily by affecting intestinal flora.188 There is a possibility that terpenoids can treat T2DM by regulating the gut flora; however, there is a lack of research into this at the present time.
1.17 Glycosides: ginsenoside's potential function in the control of gut microbiota and type 2 diabetes
1.17.1 Saponin
Due to the diversity of aglycone structures present in saponins, these compounds could be classified as either steroids or triterpenoids.189 Both these components are important parts of the active phytochemicals found in many medicinal herbs.190 Triterpenes or spirostanes, which are ubiquitous in nature, are employed to make the course of glycosides classified as saponins.191 Studies have shown that saponins have a wide variety of hypoglycaemic sites and channels, which may make them useful for promoting the recovery of damaged pancreatic cells and the enhancement of hormone actions that contribute to the maintenance of normal blood sugar levels.192 In addition, saponins have been shown to improve glucose tolerance and manage the levels of lipids in the blood. This indicates that chemicals offer a diverse set of opportunities for research and development as potential therapies for diabetes.63
Ginsenosides are the principal active components found in ginseng, which is used in traditional medicine.193 They are solely responsible for the vast array of curative properties that are possessed by the plant. Some of these effects include a reduced risk of diabetes, decreased inflammation, protection of the liver and maintenance of a healthy cardiovascular system.194-197 Ginsenoside Rg5, which was evaluated, had shown indications that it had an anti-diabetic effect.198
Research conducted in diabetic db/db mice described that ginsenosides Rg5 seemed to have the capability to alter the intestinal microbiota. There is a possibility that the main logic of ginsenosides Rg5 is to rebuild the barrier function and alleviate inflammation related to metabolic endotoxemia. Taken as a whole, pointed to the possible consequence that ginsenosides Rg5 might also fulfil the function of something like a prebiotic and efficaciously contribute to positive outcomes in the monitoring of patients suffering from T2DM.126
In order to effectively treat type 2 diabetes, it is essential to recognise the role that medicinal herbs play in improving the gut flora. However, from the perspective of modern science, more investigation is required to fully understand the theoretical foundations. The most common effects of medicinal herbs on the gut microbiota include shifts in the make-up of the intestinal flora, increases in the numbers of beneficial bacteria and butyrate levels, and reductions in the numbers of opportunistic pathogens.
The majority of evidence comes from investigations on animals, and there are fewer clinical reports available in human diabetics, so more evidence from human clinical trials is needed, and the fundamental principles still need to be probed, before actually rendering it realistic regarding the intervention techniques employing medicinal herbs for modifying the intestinal microflora in contemporary therapeutics. However, there is still optimism that this strategy will 1 day be introduced into regular clinical practice because medicinal herbs can be used to alter gut microbiota in the treatment of a wide range of metabolic illnesses, including T2DM. It is still possible that the future of medicinal herbs in the treatment of T2DM will improve after clinical trials are undertaken to standardise the use of medicinal herbs as well as precise dose requirements and related characteristics.
2 CONCLUSION
2.1 Emerging potential of medicinal herbs in modulating intestinal microbiota for preventing T2DM
An increasing body of evidence suggests that the composition of one's gut microbiota may play a crucial role in the development of diabetes.199, 200 The different mechanisms of components inherited into medicinal herbs that are utilised in the treatment of diabetes have been discussed in this article as they relate to the treatment of diabetes. Modulation of the intestinal microbial composition, which helps to reinforce the gastrointestinal mucosal barrier, synthesis of short-chain fatty acids and reduction of the LPS-induced inflammatory response are some of the mechanisms involved here.201, 202
In addition, various medicinal herbs that are used in intervention strategies for modulating the intestinal flora are also involved in the inhibition of lipotoxicity202 regulation of the gut microbial composition, the ratio of Firmicutes/Bacteroidetes and promotion of the production of gut microbiota-derived metabolites SCFAs and BAs.203 Further extended insights are required specifically targeting the understanding of the relationship that co-ordinating the dietary factors, bioactive compound pairings, mechanisms of their action on intestinal microbiota, interactions between gut bacteria, the role of microbial metabolites, host factors, and their effects on the development, prevention and treatment of type 2 diabetes in patients.
It is important to note that, according to the most recent scientific findings, there is no strong evidence to suggest that phytocompounds from medicinal herbs can completely cure T2DM alone by enhancing the microbiota in the gut, or more so by having direct impacts on T2DM. This is one of the few challenges that still exists in the routine clinical practice when considering the perfect correlation ship between medicinal herbs, gut microbiota and T2DM. Second, it is difficult to determine the sequence of the effects of herbal medicinal herb phytocomponents and gut microbiota, similar to the problem of ‘eggs and chickens’, in which it is difficult to determine which comes first: the chicken or the egg. It is not entirely clear whether inflammation is the cause of T2DM or whether gut dysbiosis is the root cause of the disease. Therefore, further research into the deep signal regulation pathways and key targets of gut microbiota as a potentially curative intervention in T2DM is required.
However, the clinical application of these herbs is still very limited in routine medical practice. This is due to the fact that many naturally occurring compounds have only been investigated using animal models or in vitro cell models, and not through clinical trials. Because of this, it is not clear what the right dose is and how well it works to treat diabetes in humans. Still, more in-depth research is required with more clinical trials conducted in the T2DM patients and also the improved regulatory influence of intestinal bacteria over healthcare is commensurate with ‘holistic approach of’ to provide an entirely new viewpoint for further study of the scientific definition of ‘healing diverse diseases by employing the similar methodology’. The overall mechanism of gut dysbiosis, its relation to T2DM, and intervention strategies of gut microbiota utilising the phytocomponents from the medicinal herbs and the overall conclusion in summary would be promising. Consideration of phytocomponents probably would be a potential challenging therapeutics in the routine T2DM treatment in the clinical practice.204
AUTHOR CONTRIBUTIONS
The concept for the manuscript was initiated by Shabbir. The draft of the document was written by Bharati and Kadamb. Shabbir assisted with the search for literature. The models were created by Kadamb. The final submitted version of the article was approved by all authors.
ACKNOWLEDGEMENTS
The Editor-in-Chief and Editorial Board's invitation to prepare this manuscript and the provision of the funding for APC are greatly appreciated by all the authors.
CONFLICT OF INTEREST
The authors declare that that there is no conflict of interest regarding the publication of this review.




