Left main coronary artery aneurysm occlusion presenting as myocardial infarction with cardiogenic shock: Patient saved by securing left anterior descending artery at cost the of other vessels
Key Clinical Message
Coronary artery aneurysms and ST-segment elevation myocardial infarction are rare in clinical practice, presenting a management challenge. To the best of our knowledge, this case appears to be the first successful percutaneous treatment of a completely obstructed aneurysmal left main coronary artery.
1 INTRODUCTION
An aneurysm in the left main coronary artery (LMCA) is a very rare finding occurring in 0.1% cases as reported by Topaz et al.1 It is unusual for a ST-elevation myocardial infarction (STEMI) to be caused by a LMCA aneurysm.2 The pathogenesis of STEMI in aneurysms is perplexing, and its therapy is still debatable. Case studies have been used primarily to describe pharmacological therapy, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), and hybrid approaches depending on the severity of coexisting coronary artery stenosis.
2 CASE HISTORY/EXAMINATION
A 40-year-old male smoker and tobacco chewer presented to the emergency department 8 h after onset of resting angina. The patient's history was unremarkable without any record of similar symptoms experienced earlier. He also did not have a family history of ischemic heart disease.
3 DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
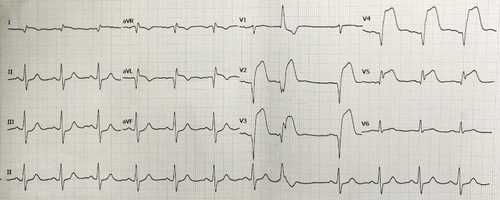
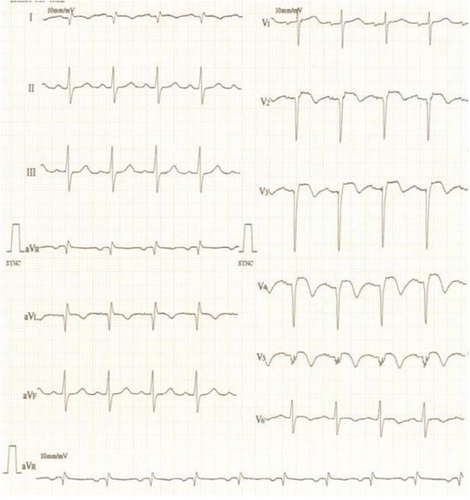
His physical examination revealed a heart rate of 116 beats/min, blood pressure of 90/66 mmHg, respiratory rate of 20 breaths/min, and oxygen saturation (SPO2) of 88%. Electrocardiography showed ST elevation in V2-V6, I, and aVL, suggestive of anterior wall myocardial infarction (AWMI) (Figure 1). 2D echocardiogram showed severe hypokinesia of the left anterior descending artery (LAD) and left circumflex artery (LCx) territory with 30% left ventricular ejection fraction (LVEF). There was no evidence of a LMCA aneurysm on echocardiogram. During hospitalization, the patient developed hemodynamically unstable ventricular tachycardia (VT) which was treated with DC cardioversion. He subsequently also developed hypotension requiring ionotropic support (noradrenaline and dopamine) and was immediately rushed to the cath-lab for primary PCI.
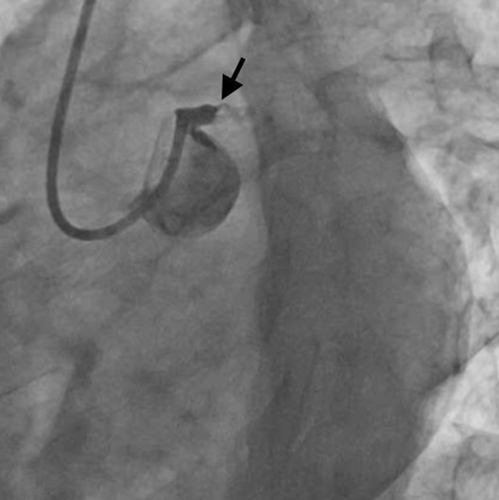
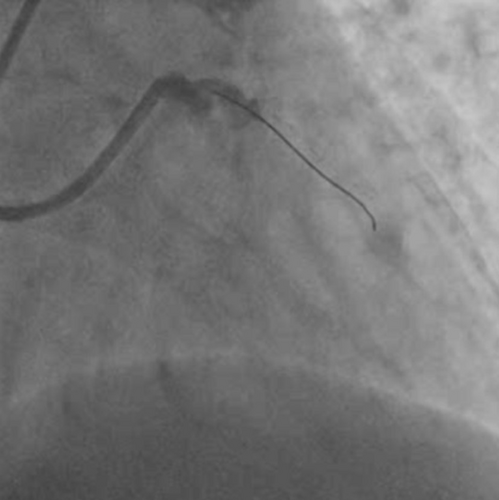
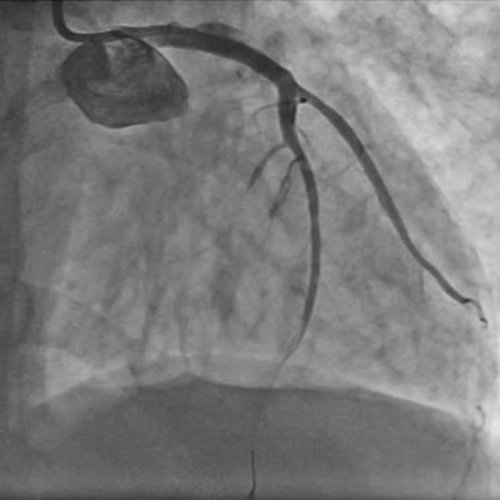
After engaging the LMCA with a 6F EBU 3.5 Launcher guiding catheter (Medtronic Inc, Minneapolis, USA) via the right radial route, an occlusion within the LMCA was encountered. Initial considerations included the possibility of a dissection induced by the guide catheter or a complete thrombotic occlusion of the LMCA, given the absence of observable branches or distal vasculature beyond this occlusion point (Figure 2 and Video S1). Due to complaints of persistent chest pain by the patient, cautious advancement of the workhorse guide wire Runthrough (straight tip, distal hydrophilic coating, Terumo Corporation, Japan) was performed, leading to an extension of staining. Finally, the lesion was crossed using a Fielder FC guidewire (polymer jacket and hydrophilic wire, Asahi Intecc, Aichi, Japan) originally intended for wiring the LAD and achieving some flow (Figure 3), but ultimately went into a diagonal branch (Video S2). As the thrombo suction catheter was unable to cross, the LMCA lesion was pre-dilated with a 2 × 10 mm semicompliant balloon.
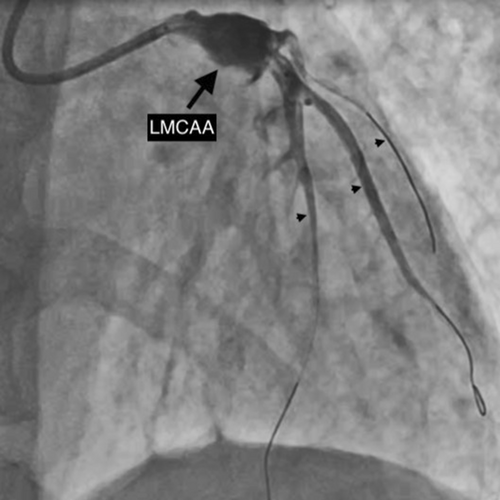
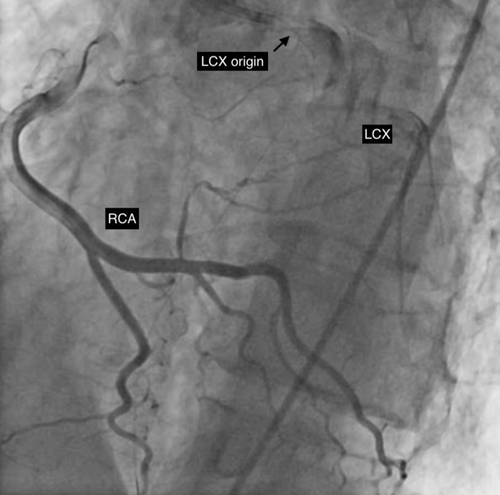
After establishment of partial blood flow within the LMCA (Figure 3), a 6 × 10 mm aneurysm was discovered in the LMCA (Figure 4 and Video S3). The case was discussed among the cardiothoracic surgical team. The decision was made to pursue revascularization using PCI. Two other Fielder FC wires were introduced into both the LAD and the ramus intermedius (RI). Due to high probability of slow flow/no flow, glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitor injection was given. Dilation of the RI was also performed with a 2 × 10 mm semicompliant balloon (Figure 5 and Video S4). While both LAD and RI achieved thrombolysis in myocardial infarction (TIMI) grade I flow, efforts to wire the LCx were unsuccessful (Video S5). Consequently, a decision was made to employ a 5F JR diagnostic catheter (Medtronic Inc, Minneapolis, USA) via the right femoral route to perform an angiogram of the right coronary artery (RCA). This imaging revealed the RCA was normal; however, a grade 3 retrograde collateral circulation was evident from the RCA to the LCx, suggestive of LCx chronic total occlusion (CTO) (Figure 6 and Video S6).
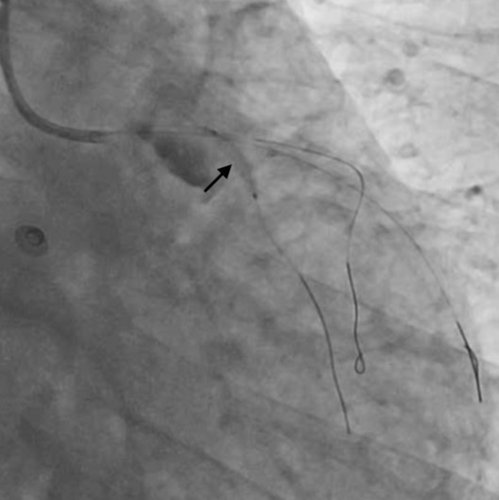
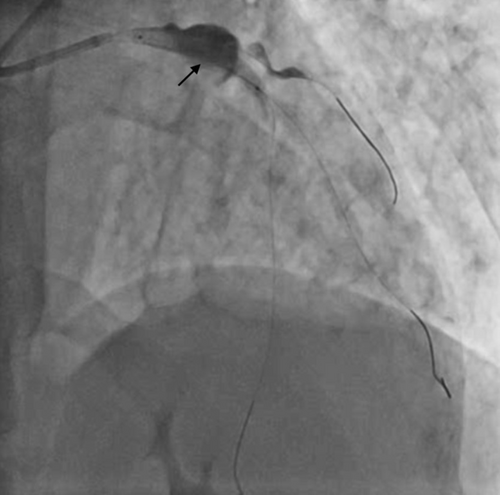
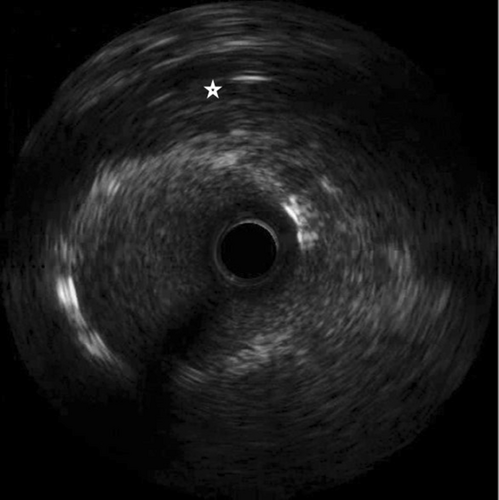
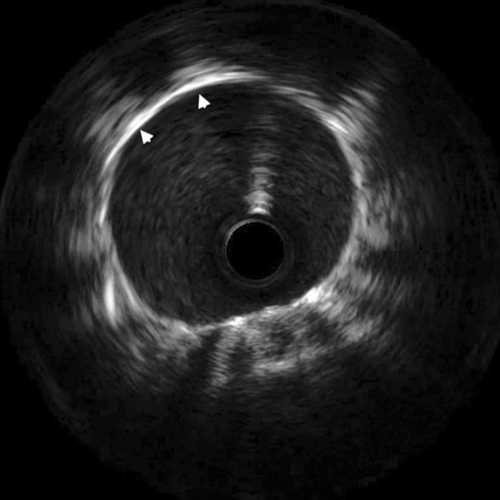
Our intended approach involved the implantation of a covered stent in the LM-LAD. However, due to the delayed availability of the designated stent and the patient's compromised hemodynamic state, a 3.5 × 20 mm Promus Elite stent (Everolimus Eluting Platinum Chromium Coronary Stent System, Boston Scientific Corporation, Marlborough, USA) was deployed from LMCA to LAD (Figure 7 and Video S7). Subsequently, stent implantation a 4 × 10 noncompliant balloon was employed for postdilatation. This yielded improved blood flow in the LAD and diagonal. Notably, on intravascular ultrasound (IVUS) imaging, the LM aneurysm was visualized as a radiolucent shadow with layers and scintillating blood shadow surrounding the stent struts and the stent appeared to dangle, creating a potential nidus for thrombus formation (Figure 8 and Video S8). In an endeavor to exclude the aneurysm, a 3.8 × 26 mm Graftmaster covered stent (Coronary Stent GraftSystem, Abbott Vascular, Santa Clara, USA) was subsequently deployed from ostial LMCA, encompassing the LCx and small sized RI extending up to the LAD/diagonal bifurcation (Video S9) and postdilated with a 5 × 8 mm noncompliant balloon.
4 OUTCOME AND FOLLOW-UP
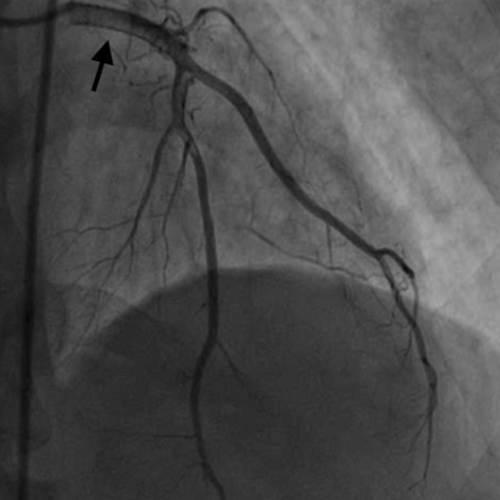
Finally, TIMI II flow in both LAD and diagonal was achieved (Figure 9 and Video S10). Post procedure IVUS revealed bright echo reflection due to the covered stent and echo drop out was observed behind the multiple layers of stents (Figure 10 and Video S11). Ionotropic agents were gradually tapered off after PCI and stopped on the second day of the procedure. The patient was discharged in a hemodynamically stable condition on aspirin, clopidogrel, rivoraxaban, and high dose statin. Triple therapy was planned for 6 months as per the high ischemic risk as an individualistic approach due to lack of any definite set of guidelines for medical management of coronary artery aneurysm. At 3 month follow-up, ECG showed qS pattern with ST segment elevation and T wave inversion in V2-V5 and qS pattern in I, avL. (Figure 11). 2D- Echocardiography showed improved LVEF of 40%. The patient's symptoms improved. Angiography revealed TIMI III flow into the LAD and its branches (Figure 12 and Video S12) and complete obliteration of the aneurysm. Coronary tomography (CT) imaging was not performed at follow-up. Ultrasound abdomen and Carotid Doppler were done and there was no evidence of aneurysm at any other site. The patient was also screened for autoimmune diseases, but the panel was unremarkable. The lack of any other clinical features suggestive of multisystemic involvement and evidence of CTO of LCx is suggestive of atherosclerotic coronary artery disease (CAD) being the most likely cause of LMCA aneurysm in our patient.
5 DISCUSSION
The term “coronary artery aneurysms” (CAAs) refers to localized irreversible dilatation of a coronary artery to a diameter more than 1.5 times that of the adjacent normal coronary segments. In contrast, coronary artery ectasias (CAE) occur as widespread arterial dilations wherein the length of the dilated segment is more than 50% of the diameter.3, 4 Morgagni published the first pathological description of a coronary artery aneurysm.3 With the advanced application of CT and invasive coronary angiography, an anticipated increase in detection rate of CAAs exists.
Although the exact prevalence of CAAs is difficult to determine due to their asymptomatic nature, it is likely underestimated—reported prevalence ranges from 0.3% to 5%. RCA is most affected (40%–70%), followed by the LAD (32.3%) and LCx (23.4%).4, 5 A review recently published by Negro et al. identifies atherosclerosis (40%) as the most common cause of CAAs, followed by inflammatory (Kawasaki disease and Takayasu arteritis) (12%) and congenital (9%) factors. Other rare causes include infections (bacterial, fungal, mycobacterial, lyme, syphilitic, septic emboli, mycotic aneurysm, and HIV), connective tissue disorders (Marfan syndrome and Ehlers-Danlos syndrome), and traumatic (post PCI).6
Compared to stable angina (43%) and acute coronary syndromes (ACS) (32%), which were more often the first clinical signs, 14% of CAA were incidental observations. 18.6% of the patients presented as STEMI.
While most patients are asymptomatic and are diagnosed incidentally, symptomatic patients often present with either effort angina or ACS owing to concurrent obstructive atherosclerotic disease or local thrombosis and embolisation.7, 8 Rarely, it can present as cardiac tamponade due to CAA rupture or symptoms due to compression of adjacent structures. CAAs might even manifest as an incidental anterior mediastinal mass and syncope.9, 10 Occasionally, a systolic murmur can be detected over the precordial region.3
The most effective diagnostic approach for CAAs likely involves coronary angiography coupled with IVUS. IVUS can help address drawbacks such as underestimation of CAA size due to intraluminal thrombi and differentiation between true and false aneurysms. Transthoracic echocardiography (TTE) is particularly helpful in the detection of abnormalities in the proximal LMCA and RCA, especially in children with Kawasaki disease. CT angiography is valuable for assessing larger CAAs and those with saphenous vein graft aneurysm. Optical coherence tomography (OCT). OCT capabilities are constrained by the infrared scan's narrow diameter.7, 8
Given the rarity of this cardiac entity and the lack of standard treatment guidelines, management approaches typically involve anticoagulation-based medical therapy, stenting using covered stents, or surgical intervention. Surgical management in form of aneurysm ligation and CABG requires a planned approach and is mostly done for stable coronary artery aneurysm patients with CTOs, long calcified and bifurcation lesions. However, hemodynamically unstable patients or patients presenting as ACS have been frequently treated by percutaneous revascularization. Literature suggests that overall, there were no differences on aneurysm complications, unstable angina, infarction, embolism, stroke, bleeding, major cardiac events, or death either regarding CABG versus PCI, but higher rates of heart failure were found in the CABG group.12 Smaller CAAs (<10 mm) are usually managed by covered stents. Covered stents are usually bare metal stents. The use of covered stents for CAAs has been challenging and there is only small data available. The risk associated with the use of covered stents incudes restenosis or thrombosis, lack of flexibility, and sealing of side branches.11 Hence, treatment decisions should be tailored to patient characteristics, clinical presentation, and the location and morphology of the aneurysms with individualized approach and in accordance with the cardiac surgery team.
In the present case, as the patient suffered an acute AWMI with a completely occluded LMCA, it was decided to assess the anatomy of the LAD and LCx and then pursue revascularization. A collaborative discussion was held among the Cardiac Surgery Heart Team and it was agreed that the patient was at high surgical risk as he was in cardiogenic shock. Thus, PCI was decided upon as a lifesaving strategy. A planned approach to cross the lesion and wire the LAD resulted in inadvertent wiring of the diagonal artery. After discovering the LMCA aneurysm after predilation, another wire was placed in the LAD and RI; however, wiring and visualization of LCX proved unsuccessful. Consequent RCA angiography revealed complete LCX occlusion, filling retrogradely through the RCA. It was decided to stent LM-LAD up to LAD and the diagonal bifurcation with a covered stent. Covered stents in such a LMCA aneurysm have a high chance of stent thrombosis. Medical management of coronary artery aneurysm has no definite set of guidelines. Literature suggests use of triple therapy and dual antiplatelet therapy (DAPT) in high ischemic risk patients with atherosclerotic CAD. CAAR registry suggested that most advanced stages of the disease like aneurysm with multi vessel disease, could have a benefit with prolonged antiplatelet strategy or anticoagulation. Considering the clinical profile, the cardiovascular risk burden and most frequent presentation (ischemic), such cases should be managed as aggressive CAD with high ischemic risk.12 In our patient there was a giant coronary artery aneurysm with large thrombus burden and findings suggestive of atherosclerotic CAD, so we preferred to keep him on triple therapy for 6 months, followed by DAPT for 12 months. At the 3 month follow-up, the patient's symptoms improved, and angiography revealed TIMI III flow and completely obliterated aneurysm. To the best of our knowledge, this case appears to be the first successful percutaneous treatment of a complete occlusion of aneurysmal LMCA.
6 CONCLUSION
LMCAAs can arise due to a variety of factors, the specific underlying cause in the present patient is most likely atherosclerosis. Treatment options typically encompass anticoagulation to mitigate the risks associated with thrombus formation, and surgical intervention is often the preferred approach. However, in the case of ACS and unstable patient, PCI becomes a viable choice if emergency bypass grafting is not feasible. Nevertheless, if the LM is implicated, a hybrid approach may be considered, especially if all vessels are unable to receive a bypass graft.
AUTHOR CONTRIBUTIONS
Sharad Chandra: Conceptualization; data curation; writing – original draft; writing – review and editing. Rajeev Krishna Choudhary: Data curation; writing – review and editing. Ayush Shukla: Data curation; writing – review and editing. Gaurav Chaudhary: Data curation; writing – review and editing. Somya Mahalawat, Saurabh Nagar, & Akhil Sharma: Revisions; editing and final review of the manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available from the corresponding author upon reasonable request.




