Poly-D,L-lactic acid polymer and cochlear implantation
Abstract
This case report describes a peculiar and innovative fixing procedure with a Poly-D,L-lactic acid (PDLLA) polymer in the unusual case of magnet dislodgment and rupture of the cochlear implant (CI) silicone sheath holding the magnet.
1 INTRODUCTION
Poly-D,L-lactic Acid (PDLLA) is a biodegradable and biocompatible random amorphous copolymer, composed of a mixture of D and L-isomers of polylactic acid. It has been used either as a vehicle for drug delivery, or in dentistry and in craniomaxillofacial surgery for bone regeneration and tissue engineering (pe. refining the facial contours after traumatic injuries).1 Its peculiar characteristics are represented by an extended resorption time (approximately from 5 to 6 months up to a complete reabsorption at 12–15 months) and by unique physical and mechanical properties: it is prepared on the operating table as a malleable material that can be adjusted to the surgical needs and that solidifies after a few minutes, becoming suitable to screw fixation to the skull.1
2 CASE PRESENTATION
We report the case of a 4-year-old boy who had received a simultaneous bilateral cochlear implant (CI), because of a progressive profound bilateral sensorineural congenital hearing loss of genetic origin. His medical history is remarkable because in January 2020, 1 year after CI surgery, he developed a post-viral acute disseminated encephalomyelitis (ADEM). At that time, the surgical removal of the magnet bilaterally was done to confirm ADEM by brain and spine magnetic resonance imaging (MRI); the magnets were replaced, uneventfully 2 months later.
Two years after CI, the boy incurred in a head trauma in the left temporal region on the receiver–stimulator (R/S) area of the left CI, which caused the dislodgment of the magnet and the rupture of the silicone sheath of the magnet encasement.
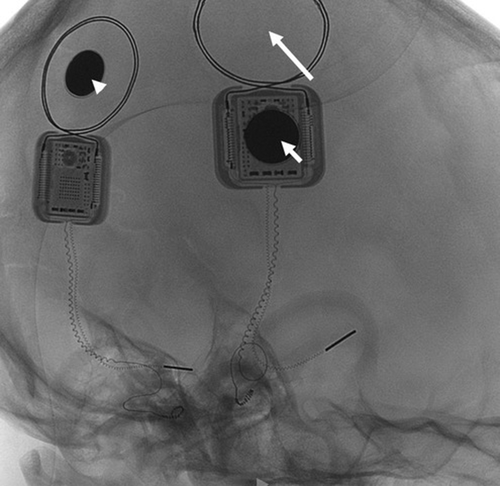
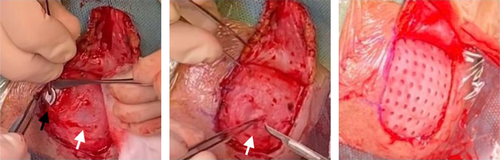
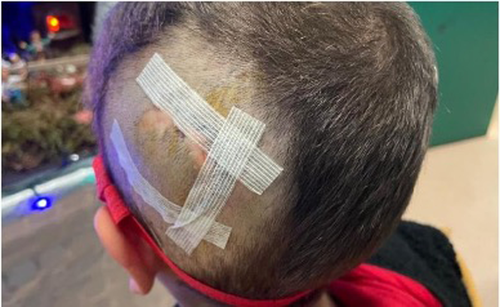
Immediately after the trauma (October 2020), the left CI speech processor could not be linked to the internal R/S. A CT scan excluded fractures of the left temporal bone but identified the dislocation of the magnet. (Figure 1) The CI team discussed with the parents the options of explanting and re-implanting the left CI: however, considering the correct position of the intracochlear array, the previous normal intraoperative impedances and neural telemetry testing and the very good speech perception performances, a conservative surgical approach was considered mandatory. The risk of a subsequent relapse of the magnet dislocation caused by the possible weakening or even rupture of the thin silicone ring around the magnet well was likely. The literature was not helpful in this respect: as far as we know, no similar cases were reported in the literature, neither surgical options different from explantation, were offered. PDLLA was identified by our maxillo-facial surgery consultant as a possible means to reduce the risk of recurrent dislodgment. After thorough discussion and informed consent by the parents, 2 weeks after the head trauma, the boy underwent a surgical revision. A small skin incision over the R/S was performed and 1 cm of the cortical bone around it for was expose. (Figure 2) The dislodged magnet was replaced with a new sterile one, and a tiny fissure of the silicon ring was observed. In order to reinforce the damaged silicone case, a PDLLA mesh was prepared: first, it was heated in hot water, then, it was shaped to correctly cover the recess, and finally, it was screwed to the bone using five dedicated Sonic Pins®, inserted in pre-drilled holes, by means of the SonicWeld® system. As shown in Figure 2, PDLLA has been applicated as a coating over the CI silicone case and fixed to the bone; the insertion was done with a “sonotrode”, a dedicated ultrasonic tool that liquifies and expands the pins after the insertion, allowing a tight bond with the mesh and anchoring them securely to the holes in the bone. No complications were noticed immediately after the surgery. Figure 3 shows the surgical site without inflammation, edema, hematoma, or infection at 1 month after surgery; the area above the R/S was thickened but no coupling troubles were reported at the time of CI reactivation. Immediate restoration of optimal auditory performances at the pre-trauma levels was achieved. At the latest check-up (21 months), no complications have been observed, the CI is working regularly, and the child has no complaint.
3 DISCUSSION
Bioresorbable and biodegradable osteosynthetic fixation plate PDLLA systems offers several advantages over the traditional osteosynthesis with non-biodegradable materials (such as titanium meshes, Gore-tex implants, malleable ceramics, and acrylates), including the absence of corrosion and metal accumulation in tissues and of removing the implants after osseous healing; the ease of molding it to the requested shape by hand after softening in hot water for a few minutes; the chance to further trim it by a drill with a soft diamond bur when hardened (quickly, after 10–15 min); the absence of inflammatory or foreign body reactions or granuloma formation, owing to its optimal bio-compatibility. Histological studies demonstrated that PDLLA determines a local fibrous tissue growth that tightens the bond to bone and to the surrounding tissues, including synthetic implants. The bonding strength is guaranteed for 8–10 weeks.2, 3 The disadvantages are the maximum load tolerance, which is lower than metallic meshes, and allergic reaction in sensitive subjects (a skin patch test is recommended in allergic patients).
Magnet extrusion or migration is more likely after CI revision surgeries owing to skin-muscle flap devascularization or atrophy related to repeated coagulation, scarring, infection, or foreign body reactions.4 Therefore, a resorbable material, stiff enough to prevent the magnet extrusion in the short term, and that could be stable enough in the long term, possibly being replaced by a thick fibrous layer avoiding exposure and/or ulceration was needed. Since there were no reports in the literature, we were concerned about possible tissue reaction to PDLLA: a fibrous encapsulation of the PDDLA mesh might have been causing an excessive thickening of the soft tissues over the R/S, impeding a correct coupling of the external speech processor and, on the contrary, a rapid biodegradation might have provoked a new magnet dislocation soon after revision surgery.
4 CONCLUSIONS
The number of CI recipients is rapidly increasing worldwide and the risk of incurring in head traumas while wearing the sound processor is higher in small children.5 The surgical repair with the PDLLA mesh might be considered in the surgical repair to avoid explantation and re-implantation of the CI in the cases with an intact skin and a rupture of the thin silicone ring around the magnet.
AUTHOR CONTRIBUTIONS
D.R. and B.G involved in conceptualization. D.R. and D.Z. contributed to methodology. S.C. involved in speech perception data and follow-up. G.C. performed neuroradiological data. F.M. involved in writing the manuscript. F.D.B. involved in writing, review, and editing of the manuscript. F.D.B., L.P. and D.Z. involved in supervision. All authors have read and agreed to the published version of the manuscript. The authors have contributed substantially to the work reported.
ACKNOWLEDGEMENT
The authors are grateful to the family of the patient and to Dr Giorgio Lilli for Figure 2 intraoperatively capturing. Open Access funding provided by BIBLIOSAN.
FUNDING INFORMATION
This paper is not published elsewhere and has not simultaneously for publication elsewhere. Authors have no relationship with commercial companies. No funding source supported this research.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ETHICAL APPROVAL
The parents of the patient signed an informed consent for the use of his images and clinical history for scientific and educational purposes. The Institutional Review Board of Fondazione Ca′ Granda Ospedale Maggiore Policlinico approved this study.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.