Fontaine progeroid syndrome—A case report
Abstract
Fontaine progeroid syndrome (FPS) is an autosomal dominant condition caused by pathogenic variants in the SLC25A24 gene. Eleven cases have been described in the literature, with early lethality in some. We discuss the clinical course of a patient from birth until his death at 7 months.
1 INTRODUCTION
Fontaine progeroid syndrome (FPS) is a rare, autosomal dominant condition caused by pathogenic variants in the SLC25A24 gene. To date, 11 cases have been described in the literature, in each patient the pathogenic variant arose de novo. Early lethality has been described in some patients with FPS.
We discuss a patient born in Ireland with a constellation of congenital anomalies consistent with the diagnosis of FPS and compare his phenotype to the previously reported cases. His diagnosis was confirmed with the finding of a de novo heterozygous pathogenic variant in SLC25A24, p.(Arg217His). We discuss his clinical course from birth until his death at 7 months from pulmonary hypertension and multiorgan failure, exacerbated by human rhino/enterovirus.
2 CASE
This patient was born in a tertiary maternity hospital at 37 weeks gestation by vaginal delivery following induction of labor. The patients Apgar scores were 9 at 1 min and 9 at 5 min of life. His birth weight was 1.68 kg and his head circumference 29.4 cm, both below the 0.4th centile.
Following spontaneous conception in this pregnancy, the patient's mother was referred to a tertiary specialist maternity service from a regional maternity unit due to intracranial and genital abnormalities on antenatal ultrasound at 29 weeks gestation. There were no reported antenatal insults or teratogenic influences. A subsequent fetal MRI performed at 31 weeks showed structural abnormalities, possible schizencephaly or subcortical heterotopia, severe symmetrical IUGR, and oligohydramnios. No antenatal genetic investigations were performed.
The patient had severe pulmonary hypertension in the early postnatal period, requiring CPAP-associated cardiorespiratory anomalies included a small right sided pneumothorax, severe tricuspid regurgitation with impairment of right ventricular contractility, and a patent ductus arteriosus.
The patient had poor central tone from Day 1 of life. Postnatal microarray, performed on Day 1 of life, demonstrated a loss of approximately 71 kb at 16p13.3 with no other abnormalities. This loss was within the OMIM non-morbid gene RBFOX1, OMIM #605104.1 but was not thought to be relevant to the patient's phenotype. This loss was also demonstrated in the patient's unaffected father.
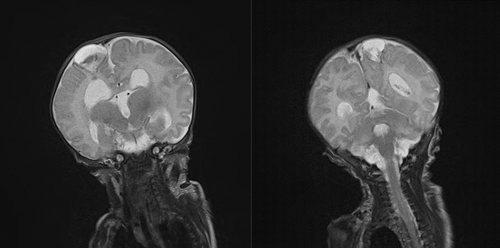
An MRI brain performed on Day 31 of life demonstrated cystic structures in the parietal lobes bilaterally (Figure 1a,b) with a Dandy–Walker malformation. There was no evidence of schizencephaly on postnatal MRI. No neurosurgical intervention was required at this time. The patient had echocardiographic evidence of pulmonary hypertension. Initially, it was felt that his degree of pulmonary hypertension was not compatible with life and given the concerns for significant neurological impairment, he was transferred to a regional pediatric center with palliative intent. Due to improving clinical status, however, the patient was well enough to be discharged home with nasogastric feeding and intermittent home oxygen after 41 days.
At 5 months of age, the patient was admitted electively to a tertiary pediatric center. Trio whole exome sequencing was performed. He was found to have a de novo heterozygous pathogenic variant in SLC25A24 p.(Arg217His), confirming the diagnosis if FPS. An echocardiogram was performed and showed the degree of pulmonary hypertension with right ventricular hypertrophy had improved and the tricuspid regurgitation had resolved. The cause for his pulmonary hypertension was not clear. There was no evidence of a primary cardiovascular or respiratory cause and was likely neurological in origin. Additionally, the patient was found to have anemia [Hb 71 g/L (reference range 105–135 g/L)] with a leucoerythroblastic blood film. No evidence of hematological malignancy was detected by flow cytometry. In view of the progeroid features, telomere lengths (TL) were measured on peripheral blood and showed that TLs were low in lymphocytes and granulocytes (between 1st and 10th centiles). TL in lymphocyte subsets were normal (between 10th and 90th centiles) in naive T cells and B cells, they were very low (below 1st centile) in memory T cells, and low in NK cells.
A cranial ultrasound was performed which showed communicating hydrocephalus for which a ventriculoperitoneal (VP) shunt was inserted.
At 7 months of age, the patient suffered acute deterioration requiring pediatric intensive care support. Evaluation revealed severe pulmonary hypertension. Human rhino/enterovirus infection was detected which was thought to have exacerbated worsening pulmonary hypertension. Despite intensive care support, his pulmonary hypertension was inotrope refractory, multiorgan failure ensued, and he died a short time later.
3 DISCUSSION
Fontaine progeroid syndrome (FPS) is caused by heterozygous pathogenic variants in the SLC25A24 gene. Inheritance is autosomal dominant, and penetrance is unknown.2 It is a rare disease, affecting fewer than 1 in 1,000,000 live births.3, 4 Prior to the association with SLC25A24 patients with FPS were thought to have one of two congenital progeroid syndromes; Gorlin-Chaudhry Moss Syndrome (GCMS), first described by Gorlin et al.5 in 1960 and Fontaine Farriaux Syndrome (FFS) first described in 1977.6 Historically, FFS and GCMS were considered separate entities.
Pathogenic variants in SLC25A24 have been found in patients clinically diagnosed with both GCMS and FFS. The term fontaine progeroid syndrome is now preferred. Those cases previously described as GCMS likely represent a milder form of FPS.2
To date, all reported cases of FPS have arisen de novo.7, 8 Only 11 cases of FPS have been described in the literature with a confirmed pathogenic variant of SLC25A24.3, 4, 7, 8 Patients with phenotypic overlap have been described, but without a confirmed genetic diagnosis.5, 9-11
The SLC25A24 gene was mapped to chromosome 1 by genomic sequence analysis in 2004.12 It is located at 1p13.3. SLC25A24 encodes for ATP-Mg/phosphate carrier 1 APC1.13 This carrier transports ATP-Mg in exchange for intra- or extra-mitochondrial phosphate.14 It is involved in regulating the matrix adenine nucleotide pool. The disease-causing missense variant identified within this gene is caused by the mutations c.650G>A (p.Arg217His) and c.649C>T (p.Arg217Cys).7, 8 The variant is hypothesized to be a pathological gain of function variant.7, 8 It is unclear to date how pathogenic variants in SLC25A24 cause the symptoms of FPS. There have been no animal models eliciting pathogenic mechanism of variants in SLC25A24. Writzl et al. demonstrated markedly altered mitochondrial morphology and function resulting directly from the disease-causing variants in SLC25A24 in fibroblasts.8 They hypothesize that SLC25A24 pathogenic variants impair mitochondrial ATP synthesis and cause hyperpolarization and increased proton leak in association with altered energy metabolism.8 Ehmke et al.7 also demonstrated abnormal mitochondrial morphology in fibroblasts in response to oxidative stress. They hypothesize that variable progeroid appearance arises due to aberrant development of skeletal, fat, and connective tissues. The exact underlying mechanism causing the clinical phenotype has yet to be elucidated. The clinical features are numerous and are listed in Table 1.
| FPS (OMIM) (Bocchini and xxx, 2018)2 | The patient | |
|---|---|---|
| Growth | ||
| Short stature | + | + |
| IUGR | + | + |
| Head & Neck | ||
| Brachycephaly/turricephaly/microcephaly | + | + |
| Large anterior fontanelle | + | + |
| Progeroid appearance | + | + |
| Broad forehead | + | + |
| Triangular face | + | + |
| Low/Posterior anterior hairline | + | + |
| Depressed supraorbital ridges | + | − |
| Midface hypoplasia | + | + |
| Long/flat philtrum | + | − |
| Micrognathia/Retrognathia/prognathia | + | − |
| Low set/dysplastic ears/hypoplastic ears/hypoplastic absent earlobes | + | + |
| Conductive hearing loss | + | − |
| Short/down slanting palpebral fissures | + | − |
| Deep set eyes/prominent eyes | + | + |
| Hypertelorism | + | + |
| Hyperopia | + | u/k |
| Laterally up slanting eyebrows | + | + |
| Synophrys | + | + |
| Depressed Nasal root/convex nasal ridge/small nose | + | + |
| Small mouth/protruding tongue | + | + |
| Thin upper lip/protruding lower lip | + | + |
| High arched palate | + | + |
| Oligodontia/microdontia | + | u/k |
| Hypertrichosis | + | + |
| Cardiovascular | ||
| Patent ductus arteriosus | + | + |
| Tricuspid insufficiency | + | + |
| Atrial septal defect | + | − |
| Left ventricular hypertrophy | + | − |
| Bicuspid aortic valve | + | − |
| Pulmonary hypertension | + | + |
| Right ventricular hypertrophy | + | |
| Aortic ectasia | + | − |
| Respiratory | ||
| Respiratory insufficiency | + | − |
| Pulmonary hypoplasia | + | − |
| Reduced number of alveoli | + | u/k |
| Recurrent aspiration pneumonia | + | − |
| Pneumothorax/bronchopleural fistula | + | + |
| Chest/Abdomen | ||
| Widely spaced nipples/small nipples/absent nipples | + | + |
| Umbilical hernia | + | + |
| Abdominal muscle hypoplasia | + | + |
| Gastroesophageal reflux | + | + |
| Feeding problems | + | + |
| Partial Malrotation | + | − |
| Anteriorly placed anus | + | − |
| Anal prolapse | − | + |
| Genitourinary | ||
| Micropenis/scrotal hypoplasia/hypoplastic labia majora | + | + |
| Cryptorchidism | + | + |
| Skeletal/Muscle | ||
| Delayed bone age/low bone density/deficient endochondral ossification | + | u/k |
| Craniosynostosis | + | − |
| Premature fusion of coronal sutures/premature fusion of parietotemporal sutures | + | − |
| Widely open metopic suture/widely open sagittal sutures | + | + |
| Poor skull ossification | + | + |
| Scoliosis | + | − |
| Platyspondyly | + | − |
| Hypoplastic or absent pubic bones | + | − |
| Short/absent distal phalanges of hands/feet | + | + |
| Syndactyly – hands/toes | + | + |
| Muscle weakness | + | + |
| Skin | ||
| Wrinkled skin/dermal translucency | + | + |
| Reduced subcutaneous fat | + | + |
| Redundant skin | + | + |
| Deep palmar creases | + | − |
| Small/absent nails | + | + |
| Coarse/sparce scalp hair | + | + |
| Neurological | ||
| Psychomotor delay with normal outcome | + | + |
| Delayed motor development due to muscle weakness | + | + |
| Hydrocephalus | + | + |
| Hypotonia | + | + |
| Gyral simplification | + | − |
| Thin corpus callosum | + | + |
| Large lateral ventricles | + | + |
| Large posterior fossa | + | + |
| Hypoplastic cerebellum/cerebellar vermis | + | + |
| Periventricular heterotopia | + | Possible |
| Prenatal | ||
| Reduced fetal movements | + | + |
| Oligohydramnios | + | + |
| Other | ||
| Early lethality | Some | + |
| Abnormal blood film | − | + |
- Note: +, present; −, absent; U/K, unknown.
- Reference: Bocchini (2018).2
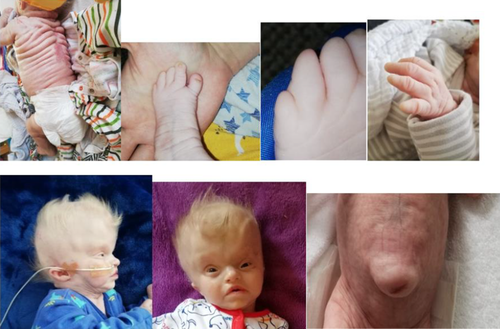
This patient's diagnosis was confirmed by the finding of a pathogenic variant in SLC25A24. This patient\s clinical features consistent with FPS are shown in Table 1 and Figure 2. They include IUGR, an aged appearance with wrinkled and translucent skin, a low anterior hairline, brachycephaly, coronal craniosynostosis, a large anterior fontanelle, triangular face with midface hypoplasia, high arched palate without a cleft, low set and abnormally shaped ears, a large umbilical hernia, microphallus with hypospadias, bilateral cryptorchidism and small scrotum, bilateral brachydactyly and clinodactyly of the hands, syndactyly of the 4th and 5th digits of the feet bilaterally, hypoplastic fingernails. Abnormal echocardiogram findings for this patient included a moderate patent ductus arteriosus (PDA) with a bidirectional shunt, right ventricular hypertrophy, severe tricuspid regurgitation, and significant supra-systemic pulmonary hypertension. PDA, atrial septal defect (ASD), and tricuspid insufficiency are described in patients with FPS. Patients with FPS who lived into early childhood often had psychomotor delay, especially gross motor delay due to muscle weakness. Ultimately, they had normal developmental outcomes.4 This patient had significant central hypotonia and muscle weakness leading to gross motor delay. Other neurological abnormalities associated with FPS includes hydrocephalus, gyral simplification, a thin corpus collosum, large lateral ventricles and hypoplastic cerebellum, and cerebellar vermis. As seen in Figure 1 this patient had an abnormal MRI brain including cystic lesions not previously described in FPS which held the differential diagnosis of porencephalic cysts, or laminar necrosis and hemorrhage secondary to an infarct or insult in utero. These lesions were also seen in this patients’ fetal MRI. He had splaying of the cerebellar vermis resulting in a dandy walker malformation and he had significant hydrocephalus requiring ventriculoperitoneal shunting.
Hematological abnormalities have not been reported in any child with fontaine progeroid syndrome in the literature. Investigations did not find evidence of leukemia as a cause for this patient's hematological abnormalities. As there have been very few patients with FPS described to date, the leukoerythroblastic anemia may represent an expansion of the phenotype. Telomere lengths (TL) are a marker of aging. In some progeroid disorders, TLs are shown to be reduced.15 TLs in FPS have not to our knowledge been reported. While the TLs are shown to be reduced in this patient, there is overlap with the normal range.
In Europe, a rare disease is defined as one affecting fewer than 1 in 2000 people.16 The care for children with rare diseases poses challenges for children and their families including the lack of knowledge and expertise within the healthcare community in their child's illness. Children with rare illnesses, and their parents, face additional challenges including late diagnosis of their child's condition, or diagnostic and prognostic uncertainty. Parallel planning, whereby plans are made for continuing life concurrently with planning for a shortened life, is increasingly being utilized to optimize the quality of life for patients with such rare conditions.17 Practical and emotional supports are thus offered on a needs basis rather than based on diagnosis or prognosis. We hope that our case report will help to contribute to the knowledge base available to those caring for patients with FPS.
AUTHOR CONTRIBUTIONS
All authors were involved in the care of this patient. The manuscript was written by S. Lally and N. Walsh. Correspondence with the patients next of kin was carried out by A. Finan and S. Lally. The photographs provided by the next of kin was sourced by A. Finan. Editing of the manuscript in its entirety was carried out by J. Kenny and A. Finan. S. Richardson and F. McElligot provided details surrounding the patients inpatient course and guidance on the manuscript, M. Cotter provided guidance in her expert area of hematology, O. Franklin provided guidance on her expert area of cardiology, J. Kenny and N. Walsh provided their expert guidance in genetics.
ACKNOWLEDGMENT
We would like to thank the patient's parents for consenting to the publication of this case report and providing photographs for referencing.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
The next of kin of the patient discussed in the case report have fully consented to the publication of the paper and have read the paper in full prior to its submission.
CONSENT
Written informed consent was obtained from the patients next of kin to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.