Successful treatment of severe post hematopoietic stem cell transplantation bronchiolitis obliterans syndrome with lung transplantation in a patient with multi-organ chronic graft-versus-host disease
Abstract
Allogeneic stem cell transplant and chronic graft-versus-host disease may lead to severe non-infectious pulmonary disease in 6% of patients at 5 years. We report the case of a young patient with acute myeloid leukemia who successfully received bilateral lung transplantation for severe bronchiolitis obliterans syndrome.
1 INTRODUCTION
Post-allogeneic hematopoietic stem cell transplantation (HSCT) bronchiolitis obliterans syndrome (BOS) is the manifestation of pulmonary chronic graft-versus-host disease (GvHD). BOS is usually diagnosed between 3 months and 2 years after HSCT.1 It occurs in approximately 5% of HSCT recipients and up to 14% of those with extrapulmonary chronic GvHD.2 Although the definitive diagnosis of BOS is based on the histological analysis of lung biopsy specimens that show peribronchiolar fibrosis and circumferential narrowing of the terminal small airway,3 a lung biopsy is rarely performed due to its invasiveness. Therefore, the diagnosis currently relies on pulmonary function testing (PFT) according to the National Institutes of Health (NIH) consensus criteria.4
Despite current treatment strategies including systemic or inhaled corticosteroids, azithromycin, and extracorporeal photodynamic therapy, patients with BOS still carry a poor prognosis.2, 5, 6 The different treatment strategies aim more toward limiting further decline of the respiratory function than reversing the obstructive defect.6-8
The role of lung transplantation (LT) in this setting is controversial. Although in many cases, it is the only treatment that could enable regaining respiratory function, its feasibility is often limited by other organ GvHD, immunosuppressive treatment, or risk of hematologic relapse.
We report herein the case of a patient with multi-organ chronic GvHD and BOS successfully treated by LT.
2 CASE PRESENTATION
A twenty-four-year-old female patient without any significant medical history was diagnosed in April 2010 with myelomonocytic acute myeloid leukemia (M4-AML) with inv(16) CBFB-MYH11 gene fusion, and FLT3 mutation. She was treated with daunorubicin and cytarabine induction therapy (3 + 7 regimen) followed by three high-dose cytarabine consolidation courses. She achieved complete cytologic remission after induction but relapsed in May 2011. Salvage therapy with gemtuzumab ozogamicin and cytarabine led to a second complete remission.
She underwent allogeneic hematopoietic stem-cell transplantation from her HLA-identical brother in November 2011 after a myeloablative conditioning regimen with cyclophosphamide and total body irradiation in second remission. Her pulmonary function tests were normal before the transplantation. Graft-versus-host disease (GvHD) prophylaxis included cyclosporine (CsA) 2.5 mg/kg daily started on Day-1 and then gradually reduced and methotrexate 15 mg/m2 on Day+1 and 10 mg/m2 on Day+3 and Day+6.
Figure 1 summarizes the follow-up after transplantation. Five months after transplantation, she experienced grade 1 acute cutaneous GvHD that evolved favorably after topical corticosteroids and a one-week course of systemic prednisolone. CsA was interrupted and Mycophenolate Mofetil (MMF) 1 g twice daily was introduced. Eight months after transplantation, she displayed multisystemic chronic GvHD with skin (grade 3), ocular, gastrointestinal (grade 2), and probable hepatic involvement with a 7-fold increase in gamma-glutamyl transpeptidase (GGT) and 2-fold increase in alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels without bilirubin elevation. Systemic corticosteroids (1 mg/kg/day) were administered, leading to significant improvement. This treatment was progressively reduced and stopped within four months.
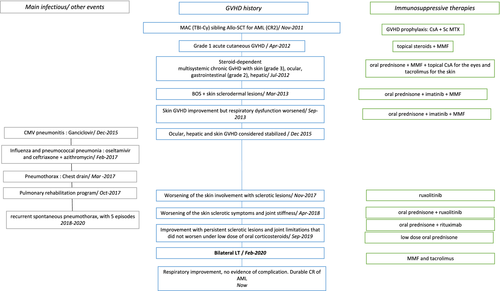
Two months later, a similar symptomatology reoccurred. Skin and liver biopsies established the diagnosis of GvHD. Prednisolone (1 mg/kg/day, 70 mg/day) was once again administered for one month before a slow dose reduction, associated with topical CsA for the eyes and tacrolimus for the skin. This enabled a complete resolution of the gastrointestinal symptoms; however, the liver abnormalities persisted and skin lesions became less inflammatory but evolved toward widespread chronic GvHD with sclerodermal lesions of the underarms.
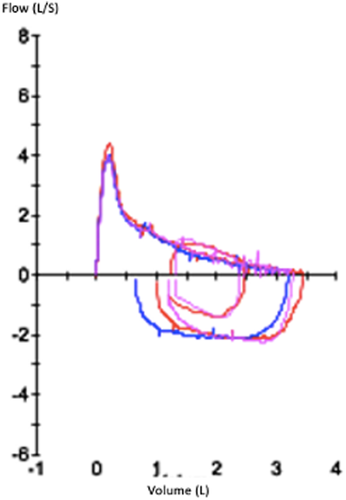
In March 2013, sixteen months after transplant, while the prednisolone had been progressively reduced to 40 mg/day, she started experiencing exertional dyspnea with expiratory bradypnea. She could walk 568 m at the 6-min walk test (predicted 731 m). The PFT (June 2013) showed a severe dysfunction with an obstructive pattern (Figure 2, Table 1), with a forced expiratory volume in 1 s (FEV1) at 41% of predicted value, FEV1/ forced vital capacity (FVC) ratio of 44%, forced expiratory flow at 25%–75% of the pulmonary volume (FEF 25–75) at 14% of predicted value. There was no reversibility using short-acting β-agonist or anticholinergics. There was significant air trapping with a residual volume (RV) of 162% predicted. She was diagnosed with BOS and was prescribed a bronchodilator treatment with budesonide/formoterol (Symbicort®) and tiotropium (spiriva®). The progressive decrease of corticosteroids was continued, and imatinib was introduced in April 2013 due to the skin involvement and joint stiffness and was continued for 1.5 year.
| Before HSCT transplant | June 2013 | November 2013 | February 2014 | November 2014 | May 2015 | April 2016 | September 2016 | October 2017 | February 2018 | January 2019 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FVC (L) (% predicted) | 3.21 (80%) | 2.71 (68%) | 3.16 (79%) | 3 (75%) | 2.77 (70%) | 2.03 (51%) | 2.07 (53%) | 1.74 (44%) | 1.48 (38%) | 1.64 (39%) | |
| FEV1 (L) (% predicted) | 1.42 (41%) | 1.06 (30%) | 1.31 (37%) | 1.27 (36%) | 1.18 (34%) | 0.94 (27%) | 0.79 (23%) | 0.73 (22%) | 0.66 (20%) | 0.70 (21%) | |
| FEV1/FVC (%) | 78% | 44% | 39% | 41% | 42% | 43% | 46% | 38% | 43% | 45% | 43% |
| FEF25-75 (L/s) (%predicted) | 0.58 (14%) | 0.42 (10%) | 0.65 (16%) | 0.54 (13%) | 0.52 (13%) | 0.52 (13%) | 0.40 (10%) | 0.32 (8%) | 0.30 (8%) | 0.34(9%) | |
| RV (L) (% predicted) | 2.52 (162%) | 2.47 (158%) | 2.21 (141%) | 2.56 (163%) | 3.04 (193%) | 3.61 (227%) | 3.62 (225%) | 3.32 (207%) | 3.30 (205%) | 2.85 (174%) | |
| TLC (L) (% predicted) | 5.87 (106%) | 5.20 (94%) | 5.40 (97%) | 5.50 (99%) | 5.77 (104%) | 5.47 (98%) | 5.68 (102%) | 5.06 (92%) | 4.75 (86%) | 4.37 (79%) | |
| RV/TLC (%) | 35% | 43% | 48% | 41% | NA | 53% | 66% | 64% | 66% | 69% | 65% |
| Corrected DLCO (% predicted) | 66% | 49% | 53% | NA | 67% | 37% | 48% | 41% | 44% | 46% |
- Abbreviations: AFVC, forced vital capacity; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FEF25-75, forced expiratory flow at 25%–75% of the pulmonary volume; RV, residual volume; TLC, total lung capacity.
In September 2013, treated by corticosteroids 5 mg/day, imatinib 300 mg/day, and mycophenolate mofetil (MMF) 2 g/day, the ophthalmic and liver involvement was stabilized, and the skin involvement became less sclerotic. However, the respiratory dysfunction worsened with a walking distance of 506 m at the 6-min walk test and a decrease in FEV1 (30% predicted at the PFT in November 2013 Table 1). Oral corticosteroids were transiently increased to 30 mg/day for this pulmonary GVHD. They were progressively decreased afterwards and finally stopped in September 2014. Throughout this treatment, she became less dyspneic and there was an improvement in FEV1 (+250 ml between November 2013 and February 2014) despite the persistence of an obstructive pulmonary disease.
In December 2015, she experienced fever and a rapid worsening of her dyspnea that was diagnosed as cytomegalovirus (CMV) pneumonitis and was successfully treated with ganciclovir. The follow-up shows a progressive worsening of the respiratory function over time afterwards (Table 1), whereas in 2016 the ocular, hepatic, and skin GvHD was considered stabilized.
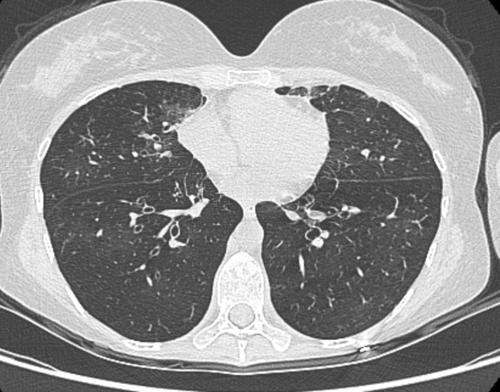
In February 2017, she was diagnosed with simultaneous influenza and pneumococcal pneumonia that required respiratory assistance. After a treatment with oseltamivir and ceftriaxone, her clinical evolution was favorable, but she required nocturnal non-invasive ventilation thereafter. Azithromycin was introduced for its anti-inflammatory effect. She remained dyspneic with minimal exertion and occasionally needed to use her nocturnal non-invasive ventilation device during the day. In March 2017, she experienced sudden-onset shortness of breath associated with sharp chest pain and was diagnosed with a large left-sided pneumothorax that evolved favorably with a chest drain. Alongside the PFT (Table 1), the results of the 6-min walk test also worsened with, in October 2017, a walking distance of 448 m with shortness of breath during the test and a drop in oxygen saturation down to 80%. Graded exercise tests measured a very altered maximal oxygen uptake (VO2 max 37% predicted). The CT-scan showed mosaic attenuation and bronchial thickening, which was consistent with the diagnosis of BOS (Figure 3). She undertook a pulmonary rehabilitation program that achieved moderate improvement, with a gain of 30 m in the 6-min walk test.
The indication of a LT was discussed, considering the severity of the obstructive defect with chronic respiratory insufficiency. The main barrier was the chronic GVHD involving other organs.
Indeed, in November 2017, a worsening of the skin involvement with sclerotic lesions of the chest, upper arms and loins, and limitation in the shoulder range of motion for abduction of 60 degrees, led to the introduction of ruxolitinib, which improved transiently her symptoms. However, in April 2018, another worsening of the skin sclerotic symptoms and joint stiffness (Photographic Range of Motion scale P-ROM 15/25) led to the reintroduction of systemic corticosteroids (1 mg/kg/day). MMF was stopped. This treatment regimen enabled a slow improvement. It was complemented with rituximab infusions in June 2018 (375 mg/m2 weekly for a 4-week course then monthly up to April 2019). In addition, the obstruction of lacrimal drainage required a surgical intervention in both eyes in 2018. Her skin became more supple, and she regained normal joint mobility in her wrists, fingers, and lower limbs whereas a slight limitation in her elbows' range of motion persisted. Her shoulders' range of motion improved despite remaining subnormal (P-ROM 19/25). Physical therapy with massages helped loosen the sclerotic lesions. Corticosteroid therapy was progressively decreased without resurgence of the symptoms. The liver function tests abnormalities did not completely correct over time but a new biopsy in 2018 concluded to iron overload. After a treatment with monthly phlebotomies, the liver tests stabilized with GGT below 10 N, ALP below 2 N, normal AST, ALT, and bilirubin.
The obstructive pattern of respiratory dysfunction became progressively associated with a restrictive pattern (total lung capacity 79% in 2019) due to the skin sclerosis. Considering the significant increase in residual volume associated with the obstructive defect, this came along with a serious loss in vital capacity (FVC 38% in February 2018). Between 2018 and 2020, she suffered from recurrent spontaneous pneumothorax, with five episodes, most of them small with mild symptoms, but one large and causing respiratory distress (February 2019). During these episodes, her nocturnal non-invasive ventilation was interrupted. This was considered a sign of the aggravation of the pulmonary involvement of the GVHD.
It was decided to postpone the LT until the rituximab could be stopped and the corticosteroids could be decreased below 10 mg/day without any resurgence of the skin GvHD symptoms. In particular, sclerotic lesions of the chest were considered an obstacle to the surgery. This was achieved in September 2019, with persistent sclerotic lesions and joint limitations that did not correct but did not worsen under this low dose of oral corticosteroids. She finally underwent bilateral cadaveric LT in February 2020 at the Bichat hospital lung transplantation center. Histopathologic analysis of the native removed lungs was not performed.
Following the procedure, she presented with probable acute cellular rejection that evolved favorably with corticosteroids, invasive pulmonary aspergillosis treated with voriconazole and nebulized amphotericin B, and pneumonia due to Pseudomonas aeruginosa treated with piperacillin/tazobactam, amikacin, and nebulized colistin. She required intensive care with mechanical ventilation through a tracheostomy until April and was finally discharged in July. Immunosuppressive treatment after the transplant included corticosteroids (15 mg/day for 3 months before progressive decrease), MMF and tacrolimus.
In August, 6 months after the transplant, her FEV had improved although it remained below normal values (1 L, 30% predicted). She could again walk for an hour and exercise on a bicycle. She initially felt dyspneic during physical efforts, which rapidly improved, probably due to deconditioning. The functional evolution is shown in Table 2. Total lung capacity however remained diminished (75% of predicted value in March 2021), possibly due to the sclerotic lesions of the chest wall. At present, she displays no evidence of complication, and she has regained the ability to perform her activities. She remains in complete remission of her leukemia, with negative minimal residual disease. We obtained signed informed consent from patient before publication.
| June 2020 | July 2020 | August 2020 | September 2020 | October 2020 | February 2021 | March 2021 | April 2021 | June 2021 | October 2021 | |
|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 | 0.85 (26%) | 0.98 (30%) | 1.00 (31%) | 0.99 (30%) | 1.11 (34%) | 1.19 (36%) | 1.28 (39%) | 1.08 (33%) | 1.15 (32%) | 1.25 (38%) |
- Abbreviation: FEV1, forced expiratory volume in 1 s.
3 DISCUSSION
The case of post-HSCT BOS associated with multi-organ chronic GvHD presented here had mostly typical features and all the 2014 NIH criteria were met, with an obstructive defect that was objectified by a decreased FEV1/FVC ratio, a decreased FEV1 that did not correct with bronchodilators, and evidence of air trapping with increased residual volume at the PFT. The occurrence of recurrent pneumothoraces is less common but has been associated with advanced GvHD.4, 9 The restrictive pulmonary function abnormalities seen in our patient are not the characteristic of BOS, but such features have been associated with thoracic-cage restriction due to sclerotic GvHD of the chest wall.4 An early onset of the BOS after HSCT has been described as a pejorative factor.5, 10-12 Our patient presented a rather late onset, with first clinical symptoms occurring sixteen months after transplant. The complete PFT from the first year after HSCT was performed in another center and could not be retrieved but were described in consultation reports as normal. Despite this late onset, our patient presented severe pulmonary dysfunction.
For patients with severe end-stage lung disease from BOS, LT is often discarded as a therapeutic option due to comorbidities, extensive chronic GvHD, risk of relapse of hematologic malignancy, and prolonged immunosuppressive therapy. Recently, it has been increasingly considered as a therapeutic option, but its place remains to be defined. In a literature review which collected more than 60 patients with post-HSCT BOS who subsequently underwent LT from case reports and case series, Soubani et al.13 showed a survival at 2 and 3 years of 88% and 79%, respectively, which was similar to other reports.14 These results were corroborated by results from a pan-European series of more than 100 allogeneic HSCT recipients who received LT,15 in which the outcomes were comparable with those observed in matched control patients with other end-stage lung diseases, with survival rates at 1, 3, and 5 years post-lung transplantation of 85%, 72%, and 67%, respectively.
However, although these studies tend to show that LT is a viable option in selected patients, the proper selection of patients eligible for LT remains ill-defined and subject to major center variability. An indicator of this variability is the disparity in the results from different series. In a case series of 13 patients, Holm et al.16 reported a low mortality rate of 15%, while Koenecke et al.17 reported a mortality rate of 33% in a case series of 12 patients, and Vogl et al.18 published a fatality rate of 57% in another case series. Most cases reported were young patients, with a median age ranging from 22 to 34 across series.13-15, 18, 19
The risk of relapse of hematologic malignancy has been frequently mentioned as an obstacle to LT. For this reason, retrospective case series reported are likely to include selected patients considered at lower risk of relapse. Nevertheless, in these studies, the incidence of relapse was low, ranging from 2.5%13 to 4%15, 17 across series. This is by far below the general relapse rate after HSCT usually reported as above 30%.20 This is probably due in part to patient selection, and in part to the fact that these patients experiencing severe GvHD probably beneficiate from Graft-versus-Leukemia effect. Overall, this suggests that the history of hematologic malignancy does not contraindicate LT.
The proper timing for LT remains controversial but is probably critical to limit the risk of hematologic malignancy relapse. The length of time between HSCT and LT differed significantly across series, with a median of 18 months in the case series by Vogl et al.,18 52 months in the study by Soubani et al.,13 69 months in the study by Greer et al.,15 and 10 years in the report by Cheng et al.14 In a comparison of patients receiving LT within and after 60 months post-HSCT, Soubani et al.13 showed no significant difference in outcomes. However, Greer et al.15 showed an adverse prognosis for LT performed within 2 years of HSCT, which tends to corroborate recommendations to avoid LT during the first 2 years after HSCT for malignant disease,21 although they do not report high rates of hematologic relapse.
Another concern about LT following HSCT is the presumed increased risk of infectious complications, related to interruption of mucosal barriers in patients with GvHD, hypogammaglobulinemia, prolonged immunosuppressive therapy, and colonization by resistant bacteria. This has been inconsistently observed in various studies, with some studies reporting rates of deadly infections that did not exceed the rates seen in other LT recipients,13 while Greer et al. reported higher rates of early fatal sepsis15 and Gao et al.19 reported higher rates of fungal infections.
Finally, the extent of extrapulmonary GvHD has been mentioned has a potential pitfall, but this has been scarcely described so far. In the case series by Holm et al.,16 the low mortality rate reported might be related to the low proportion of patients with extrapulmonary GvHD in this study.22 In their considerations in the selection of LT candidates after HSCT, Cheng et al.14 state minimal chronic manifestations of GvHD and minimal immunosuppressive need for GvHD treatment as main criteria. In the multicentric cohort study reported by Greer et al.,15 a few, but not all centers, listed quiescence of extrapulmonary GvHD as a formal criteria for eligibility to LT. Seventy percent of the patients reported in their study had experienced extrapulmonary chronic GvHD, but the extent of GvHD control before LT was not detailed. The reports of LT following HSCT in patients with extrapulmonary GvHD still present at LT are scarce.23 In this report, we have presented a case of LT successfully performed 8 years after the HSCT despite a persistent sclerotic-type chronic GvHD of the skin. Liver involvement was hard to assess in a context of associated iron overload, and gastrointestinal involvement seemed resolved. The LT was postponed until systemic GvHD manifestations remained stabilized after immunosuppressive agent discontinuation.
4 CONCLUSION
This case report and the literature review tend to show that lung transplantation is a viable treatment option for otherwise irreversible BOS following HSCT. Although the optimal timing and proper patient selection remain to be specified, this report suggests that LT is feasible despite persistent but controlled GvHD.
AUTHORS CONTRIBUTIONS
JP did the literature review and wrote the first draft of the manuscript. VC was responsible for the conception of the work. JHB managed the patient before 2016. AS, MA, NC, ALM, FB, SL, PL, VC, EL, HL, FJ, EL, HM have been managing the patient since 2016. EL and HM were involved in the critical review from pulmonologists point of view. All authors were involved in the critical review and editing of the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
We have no conflicts of interest to disclose.
ETHICAL APPROVAL
This study was performed in accordance with the Helsinki declaration. Data published anonymously.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study" cd_value_code="text