Collision lung tumor associating typical carcinoid tumor to sclerosing hemangioma
Abstract
Collision tumors associating carcinoid tumor and sclerosing pneumocytoma have rarely been reported in the literature. The clinical presentation may be challenging especially in cases showing multiple and bilateral nodules. This case illustrates the association of both tumors diagnosed incidentally and illustrates a full spectrum of neuroendocrine lesions and sclerosing pneumocytoma. The authors present the case of a 52-year-old patient presenting an abdominal pain revealing a vesicular lithiasis and multiple pulmonary nodules. Radiologic follow-up of the asymptomatic lung lesions revealed the stabilization of a left lobe lesion with a disappearance of the other lesions. A lobectomy with a mediastinal lymph node curettage was performed. The microscopic examination revealed both tumor components associating a typical carcinoid tumor to a sclerosing pneumocytoma in association to lesions of diffuse neuroendocrine hyperplasia present in the peri-tumoral parenchyma. This case illustrates radiologic, gross, and microscopic features of a rare pulmonary tumor.
1 INTRODUCTION
Collision lung tumors associating typical carcinoid tumor to sclerosing pneumocytoma are rare with less than 10 cases reported in the literature.1-4 Clinical and radiological features are nonspecific. The positive diagnosis is based on microscopic and immunohistochemical findings.
2 OBSERVATION
We present the case of a 52-year-old patient, without a particular past medical history, who presented one year ago a pain in the right hypochondriac region. After an exploration by a gastroenterologist, a vesicular lithiasis was discovered. A biliary MRI was performed and revealed an asymptomatic mass of the right lung and the left lobe. Bronchial endoscopy was normal. A first CT-scan examination revealed a 24-mm mass of the lower left lobe with some satellite nodules and focal calcifications. Other millimetric masses were reported in the right lung and in the upper left lobe. FDG PET was performed and showed abnormal uptake of the mass situated in the lateral posterior lower lobe with the other satellite nodules presenting an undetermined metabolism. Increased uptake was also noticed in some interlobar adenomegalies. A second chest CT-scan was performed after 6 months. It revealed the disappearance of the millimetric nodules situated in the right lung and the upper left lobe with the stabilization of the 24-millimeter-nodule in the lower left lobe without mediastinal adenomegalies (Figure 1A). A left inferior lobectomy with subcarinal, paraoesophageal, and interlobar lymph node curettage was performed.
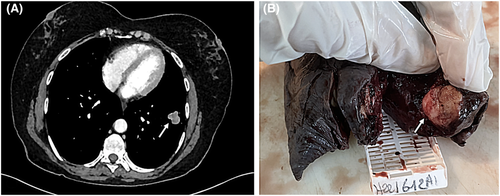
The gross examination revealed a 100x70X40 mm lobectomy with a 30-mm mass situated in the lateral posterior lower lobe with some satellite nodules measuring less than 2 to 3 millimeters (Figure 1B). The mass was located at 4 centimeters from the bronchial limit and 1 centimeter from the visceral pleura.
The extemporaneous exam concluded to a spindle cell tumor necessitating an examination after fixation and paraffin inclusion. All the tumor was studied, and many samples were made with the peri-tumoral pulmonary parenchyma.
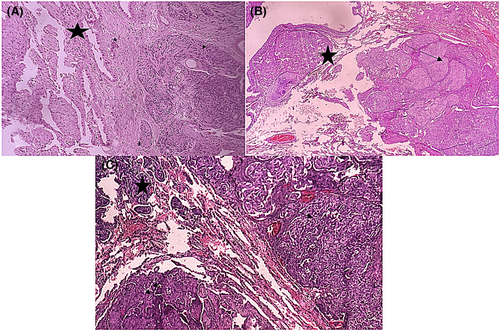
Microscopic examination revealed 2 tumor components that were intimately intricated. The first component consisted in a 20-mm spindle cell tumor organized into organoid nests with palisading arrangements surrounded by some capillaries (Figure 2A, B [arrow]). Spindle cells were regular with elongated nuclei and rare mitoses (1 mitosis/ 2 mm2) (Figure 3A). No necrosis was observed. The second component consisted in many millimetric tumoral foci made of a papillary and trabecular tumor characterized by a hyalinized stroma (Figure 2A,B [star]). Cuboidal surface cells were observed and covered the papillary structures. They were regular without mitotic figures. Within the stroma of the trabecular and papillary structures, round stromal cells were observed. Those cells were regular without mitotic figures (Figure 3B). Facing these features, the diagnosis of a collision tumor was evoked. The spindle cell tumor features were suspicious of a carcinoid tumor or a paraganglioma. The second papillary tumor was suggestive of a sclerosing pneumocytoma. The specimen performed in the peri-tumoral parenchyma revealed localized reactive neuroendocrine hyperplasia (Figure 2C,D [arrow]). This lesion was characterized by the presence of neuroendocrine bodies within the bronchiolar mucosa.
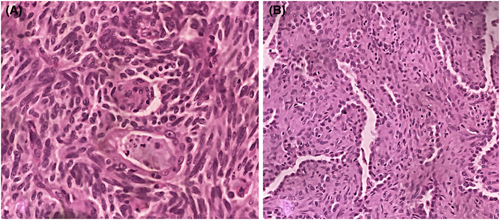
Immunohistochemical features revealed the expression of neuroendocrine markers (CD56, synaptophysin, chromogranin) by the spindle-cell-tumor (Figure 4A–D). Rare tumor cells expressed the PS100 antibody in spindle-cell-tumor. The TTF1 antibody was expressed by the spindle-cell tumor and by the surface and stromal cells composing the second tumor (Figure 4E). The cytokeratin antibody marked the tumor spindle cells and the surface cells of the second tumor (Figure 4F). The stromal cells composing the second tumor were negative.
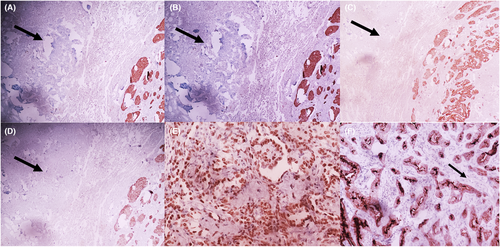
According to the morphologic and immunohistochemical features, the diagnosis of a collision tumor associating a typical carcinoid tumor and a pneumocytoma was retained. The mediastinal and interlobar lymph nodes were normal.
The patient did not present complications after a 3-month follow-up period.
3 DISCUSSION
This case highlighted the diagnostic challenges when facing collision tumors. The carcinoid tumor and sclerosing pneumocytoma have not been published as having similar origins. Carcinoid tumor takes their origin from lesions of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.2 In our case, lesions of neuroendocrine hyperplasia were reported in the peritumoral parenchyma. This fact gives a spectrum of the different neuroendocrine lesions that were present. The radiologic findings consisting in multiple and bilateral lesions are more suggestive of a diffuse idiopathic neuroendocrine cell hyperplasia. These lesions may be symptomatic or asymptomatic but present usually as multiple and bilateral masses suggestive of metastases. On the contrary, sclerosing pneumocytoma is thought to derive from primitive respiratory epithelium. The association of both tumors has been attributed to an accidental happening or an origin from multipotential stem cells. According to another theory, sclerosing pneumocytoma changes the microenvironment surrounding lung tissue and promotes pulmonary neuroendocrine cells proliferation.2 The association of carcinoid tumors to sclerosing pneumocytomas has been rarely reported on the literature.1, 3, 4 Some exceptional cases associating also clear cell adenoma have also been reported.2
Carcinoid tumors are rare accounting for less than 2% of the lung tumors. They are still classified as typical (<2 mitoses/2mm2 and lacking necrosis) or atypical (2–10 mitoses/2 mm2 and/or foci of necrosis) based on the presence or not of necrosis and the mitotic count. Sclerosing pneumocytomas are rare tumors classified among adenomas in the 2020 WHO classification of lung tumors.5 They present diverse architectural patterns, and they are characterized by the presence of both tumor cells: surface cells and stromal cells. The major differential diagnoses consisted in the paraganglioma concerning spindle cell tumor. Paragangliomas express occasionally endocrine markers, they express diffusely PS100 antigens revealing sus-tentacular cells, but they do not express epithelial markers. Sclerosing pneumocytoma may mimic adenocarcinomas but the presence of 2 tumor cells with different phenotypes allows to assess the diagnosis.5 Rare cases associating carcinoid tumor to sclerosing pneumocytoma and adenocarcinoma have been reported.1 The treatment of the association of carcinoid tumor and sclerosing pneumocytoma is based on surgical resection. The prognosis of these rare lesions has been reported to be good unless in rare cases showing exceptional pleural metastases.3
AUTHOR CONTRIBUTIONS
MM made the literature research and wrote the manuscript. AR obtained the patient consent, participated to the literature research, and performed the figures. AA, SBS, IB, and MA participated to the manuscript redaction and read the final version. FM supervised all the process and red the final version of the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The patient's privacy was respected.
CONSENT
Written informed consent was obtained from the patient to publish this case in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data are available.