Intractable low back pain during acute exacerbation of systemic lupus erythematosus
Abstract
Diagnosis of acute low back emergencies during a systemic lupus erythematosus flare necessitates high clinical suspicion and early CT.
A 71-year-old woman with low-activity SLE under prednisone 10 mg/day and hydroxychloroquine 400 mg/day presented to the emergency department with acute fever, malar-rash, and intractable low-back pain to repeated IV administration of paracetamol 1 gr, tramadol 100 mg, and parecoxib 40 mg. The patient had normal consciousness, temperature 39.1°C, MAP 65 mmHg, heart rate 94/min, and breathing rate 32/min. Laboratory tests revealed leukopenia, lymphopenia, elevated creatinine suggestive of stage-II acute renal injury, slightly elevated creatine phosphokinase, metabolic acidosis, and microscopic hematuria. Whole-body CT showed enlargement of superficial and deep muscles of right gluteal and posterior thigh compartment with decreased attenuation and massive intramuscular gas collection (Figure 1).
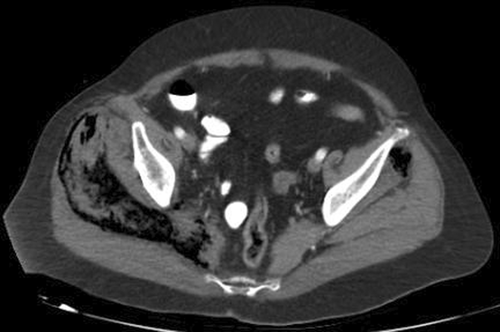
1 QUIZ QUESTION: WHAT IS YOUR DIAGNOSIS?
Diagnosis was acute extensive necrotizing myositis with associated compartment syndrome during a SLEDAI-2 K score 12 SLE flare.1 The patient admitted to ICU with SOFA Score 6. Initial resuscitation included IV administration of lactated Ringer's solutions, methylprednisolone 1 gr, empirical coverage with daptomycin 500 mg, tinzaparin 8000anti-XaIU SC, and emergency surgery including fasciotomy of right-gluteal and posterior-thigh compartment along with debridement of necrotic muscles. Despite initial management, the patient developed refractory shock requiring high dose of norepinephrine to maintain target MAP 60 mmHg, IVIG, and continuous renal replacement therapy leading to death on postoperative Day 2.2 Interestingly, urine, blood, and wound cultures proved negative, and serology revealed low-complement and high anti-dsDNA antibodies suggestive of non-infectious/immune-mediated necrotizing myositis.
AUTHOR CONTRIBUTIONS
All authors equally accessed the data and contributed to the preparation of the manuscript. BKA and HA were equally responsible for making and performing treatment decisions. HA reviewed the manuscript for critical intellectual content and had the final approval.
ACKNOWLEDGMENTS
The authors would like to acknowledge the substantial contribution of the patient for giving the written informed consent and the medical staff of our surgical department for the management of the patient.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
STATEMENT OF HUMAN AND ANIMAL RIGHTS
The present article does not contain any studies with human or animal subjects performed by any of the authors.
CONSENT
Written informed consent was obtained from the patient's family to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.