Impact of IFN-β1a in treatment of a COVID-19 patient with beta thalassemia and diabetes mellitus: A case report
Abstract
Patients with chronic diseases are severely affected by acute coronavirus syndrome. In this regard, patients with beta thalassemia intermedia and diabetes mellitus (DM) are also at high risk for coronavirus-induced respiratory failure. The present study aimed to report a case with COVID-19 with a history of chronic diseases, beta thalassemia intermedia, and DM. A 25-year-old man visited with complaints of severe shortness of breath, fever, cough without sputum, and tachypnea and admitted to the Intensive Care Unit. The patient had a history of DM, beta thalassemia intermedia, and pervious history of the splenectomy. In peripheral complete blood count (CBC diff), the number of white blood cell count was 41,100 of which 38.6% were lymphocytes. We measured the normal platelet count, hemoglobin level (9.4), and red blood cell count (3.56). ESR was 97, CRP = pos+++ and PCR was positive. The high-resolution lung CT indicated ground glass opacities in peripheral areas. The patient underwent 13 days of oxygen therapy with reservoir bag-mask, non-invasive ventilation, nasal oxygen, and pharmacological treatment with IFN-β1a and meropenem, and finally discharged with an improvement of the clinical condition. Timely initiation of treatment is very important and significant for patients with beta thalassemia intermedia with COVID-19, especially despite the underlying disease of DM. According to the present report, the use of IFN-β1a was effective as a treatment option for COVID-19.
1 BACKGROUND
New coronavirus as a cause of severe respiratory infection was diagnosed in late 2019, and it spread rapidly across countries and led to a pandemic.1 Clinical symptoms of the disease range from asymptomatic infection, mild upper respiratory symptoms to severe pneumonia with respiratory failure and death. The most common symptoms of this disease are fever, cough, pain, shortness of breath, hemoptysis, diarrhea, and increased mortality.2 The infection also leads to failure of several organs, including acute respiratory distress syndrome, acute heart damage, and shock.3 Among those people with close contact with the COVID-19 patient or take care of them, the elderly and people with a history of underlying disease and immunodeficiency are at greater risk for morbidity and mortality.4 Chronic illness may be more likely to be severely infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Some diseases such as high blood pressure, diabetes mellitus (DM), respiratory disease, and cardiovascular diseases may be associated with the pathogenesis of COVID-19.5 Furthermore, patients with beta thalassemia major with chronic conditions, including chronic blood transfusion, heart failure, pulmonary hypertension, and diabetes mellitus are at risk of COVID-19.6 Beta thalassemia major is an inherited disease in which patients are exposed to severe infections due to splenectomy, adrenal insufficiency, and high iron levels after repeated blood transfusions. Infectious complications, especially bacterial infections are common causes of death in patients with beta thalassemia major.7
2 CASE PRESENTATION
The patient was a 25-year-old man with symptoms of severe shortness of breath and a history of fever, myalgia, arthralgia, and dry cough from 5 days before of admission. The patient had a history of beta thalassemia intermedia, diabetes mellitus. He also underwent splenectomy 10 years ago. The patient was tachypnea (respiratory rare: 30) and in respiratory distress, and in the lung auscultation, crackles were heard in the lower half of both hemithorax. His Arterial o2 saturation (in room air) was 76% and he had a body temperature of 38.3°C. Arterial Blood Gas analysis showed respiratory acidosis (pH: 7.31, pco2:56, HCO3: 27.3).
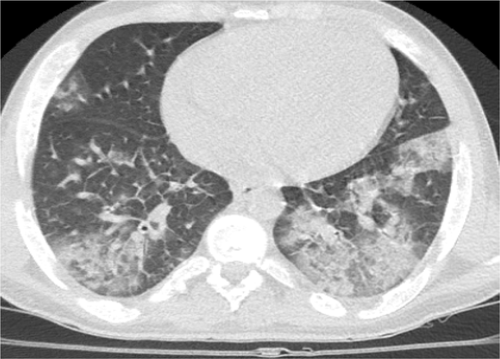
Due to the high probability of COVID-19 for the patient, PCR test was requested, and sampling was performed for the patient through the throat and nose. Initial high-resolution lung CT (HRCT) Scan showed multifocal peripheral ground glass opacities (GGO) throughout the both lungs. According to the HRCT Scan (Figure 1) and positive PCR, patient was transferred to the COVID ICU.
On the first day of hospitalization, the white blood cell count was 41,100 of which 38.6% were lymphocytes according to the peripheral complete blood count (CBC diff). Hemoglobin was 9.4, Red Blood Cell was 3.56, and platelet count was normal. ESR was 97, serum procalcitonin level = 18.3 and CRP = pos +++. Liver enzymes and BUN, Cr and serum electrolytes were normal.
From the first day of hospitalization, the patient was treated with Meropenem (1 g every 8 h) and Kaletra (lopinavir/ritonavir) (II every 12 h). Three days later, due to productive cough and continued fever, coverage of gram-positive microorganism with vancomycin (if every 12 h) was started for the patient. Due to lack of improvement in patient's clinical condition, treatment with subcutaneous injection IFN-β1a (Recigen) 44 μg every other day was started at fourth of hospitalization for five doses. The patient's blood glucose was controlled with Lantus and Novorapid insulin.
Due to hypoxemia in room air at the time of hospitalization 76%, oxygen therapy started with mask with reservoir bag (SPO2 mask with reservoir bag: 89%). On the second day of hospitalization due to the aggravation of respiratory distress and SPO2 decrement with the mask of the second day to 84%, NIV (BIPAP) supported him for 7 days. On the 9th day of hospitalization due to respiratory improvement, he underwent o2 therapy with nasal cannula for 4 days (SPO2 with nasal cannula: 97%).
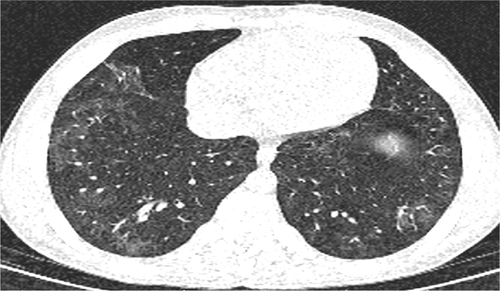
In addition, as the treatment continued, the patient's fever stopped on Day 7 and the patient's appetite improved. WBC had a decreasing trend so that the patient's white blood cell count was 14,700 on the last day of hospitalization of which 31.3% were lymphocytes. During the hospitalization period, a blood transfusion was performed for the patient on the sixth day of hospitalization. Table 1 presents the process of changing values of the ESR, CRP, serum procalcitonin level, and CBC diff test on the patient. In follow-up, the Chest CT scan in 10th day of hospitalization indicated changes for improvement peripherals GGO (Figure 2).
| Day of hospitalization | LYMPH% | NEUT% | Hb (g/dl) | RBC (106μl) | WBC (103 μl) | CRP | ESR (mm/h) | Procalcitonin |
|---|---|---|---|---|---|---|---|---|
| 1 | 38.6 | 57.7 | 9.4 | 3.56 | 41.1 | +++ | 97 | 18.3 |
| 3 | 29.4 | 66.3 | 9 | 3.39 | 52.6 | – | – | – |
| 5 | 28 | 68.8 | 8.2 | 3.23 | 32 | – | – | – |
| 6 (before of blood transfusion) | 23 | 73 | 8.1 | 3.22 | 21.5 | – | – | – |
| 6 (after blood transfusion) | 30 | 63.5 | 9.8 | 3.85 | 27.3 | – | – | – |
| 8 | 25.1 | 68.4 | 10.1 | 3.83 | 32.2 | – | – | – |
| 10 | 30.9 | 63.6 | 11.5 | 4.41 | 34.6 | – | – | – |
| 12 | 38.8 | 63.3 | 11.5 | 4.32 | 21.3 | – | – | – |
| 13 | 31.3 | 31.3 | 10.2 | 3.96 | 14.7 | + | 33 | 0.39 |
Finally, the patient in good general condition, stable vital signs, SPO2: 94% (without supplemental oxygen) was discharged on Day 13. Also, in follow-up, 2 weeks and 1 month after discharge, the patient was in good general condition and oxygenation was 96%.
3 DISCUSSION AND CONCLUSIONS
Hereditary hemoglobin disorders or hemoglobinopathy, including beta thalassemia, are the most common monogenic disorders in humans with significant involvement of several systems that require long-term treatment and follow-up. Even though there is now epidemiological evidence about SARS-CoV-2 infection in patients with beta thalassemia, the COVID-19 epidemic indicates an important challenge for hemoglobinopathy patients, family, and physicians who visit them.8
Patients with beta thalassemia are exposed to severe infections due to splenectomy, adrenal insufficiency, and high levels of iron after the frequent blood transfusions. Infectious complications, especially bacterial infections, are common causes of death in patients with beta thalassemia.7 In this regard, Abdi et al. (2005) reported an increase of 30% in infection in splenectomy patients compared with the control group because the spleen is a filter of bacteria and many invasive microorganisms and is also involved in the elimination of microorganisms that are opsonized by complement.9
People with DM are at higher risk of hospitalization and death from viral, bacterial, and fungal infections.10 It should be noted that the two-way interaction between COVID-19 and DM creates a vicious cycle in which COVID-19 leads to worsening of dysglycemia and diabetes, in turn, intensifies COVID-19.11 In this regard, Guo et al. 12 evaluated 174 patients with COVID-19 and found that COVID-19 patients with DM alone were at higher risk of severe pneumonia, enzymes secreted from tissue damage, excessive uncontrolled inflammatory responses, and anticoagulant conditions associated with impaired glucose metabolism. Furthermore, serum levels of inflammation-related biomarkers such as IL-6, CRP, serum ferritin, coagulation index, and D-dimer were significantly higher. The research results indicated that DM should be considered as a risk factor for the rapid progression and poor prognosis of COVID-19. In other words, people with DM were more prone to a severe inflammatory process that eventually led to the worsening of COVID-19. Therefore, diabetic patients should be taken into serious consideration.12
The results of a study by Kumar et al.13 on the relationship between DM and COVID-19 also indicated that a severe clinical course developed and also the mortality rates increased in patients with COVID-19 and underlying DM. Furthermore, diabetic patients with COVID-19 had a doubled mortality and more severe disease symptoms than non-diabetics.13 Mantovani et al.14 conducted a comprehensive systematic review and meta-analysis of 83 studies in 2020 and found that COVID-19 patients with DM were significantly associated with a higher risk of disease severity and in-hospital mortality. In the largest report from China, including 72,314 cases, COVID-19 patients suffering from DM had higher mortality (7.3% in patients with DM vs. 2.3% of all patients).15
Finding a cure now for COVID-19 is an urgent need. Many different treatments have been offered by different groups of physicians, and many studies have examined the production of vaccines and drugs for prevention and treatment. There are also several studies on the effect of interferon on the treatment of patients with COVID-19.16 Numerous studies have studied the treatment of IFN-I against MERS-CoV and SARS-CoV both in vitro and in vivo In combination with or without Kaletra,17, 18 ribavirin,17, 19 and Remdesivir.18, 20
Many studies have examined the effect of interferon type 1 treatment (e.g., IFN-α/β) as a potential treatment for coronavirus (COVID-19, MERS, and SARS).18, 21, 22 Type I interferons (IFN-α/β) have a wide range of antiviral activities against RNA viruses and act by inducing an antiviral response in a wide range of cell types and compatible immune response mediators. Humans produce 13 types of IFN-α and a singular IFN-β. Type I interferons finally stimulate several ISG genes (interferon-stimulated genes) that are encrypted for different types of antiviral effects. It is noteworthy that the production of IFN-β leads to a positive feedback loop that re-stimulates the expression of many IFN-α genes. Clinically, type I interferons have been approved for the treatment of certain cancers, autoimmune disorders, and viral infections (hepatitis B and C).23
In a study by Dastan et al.,3 20 patients received subcutaneous interferon beta-1a at a dose of 44 μg along with the usual treatment of Kaletra and hydroxychloroquine daily for 10 days. During the first 7 days, the fever resolved in all patients although other symptoms gradually diminished. Lung imaging studies showed significant improvement in all patients after 14 days. The research findings confirmed the use of interferon beta-1a in a combination with hydroxychloroquine and Kaletra in the treatment of COVID-19.8 Hung et al.24 studied the positive impact of the triple combination of Kaletra, ribavirin, and interferon beta to reduce the duration of hospitalization in patients with mild to moderate COVID-19.
The case study was a patient with COVID-19 with a history of DM and beta thalassemia intermedia, who had severe respiratory distress and decreased SPO2 at the beginning of the disease, but he was discharged after treatment with Kaletra and IFN-β1a, for 13 days and respiratory support with NIV (BIPAP) with SPO2 96% for 5 days and without respiratory distress. Since patients with beta thalassemia are exposed to severe infections due to splenectomy, adrenal insufficiency, and high levels of iron after the frequent blood transfusions. Therefore, timely treatment is very important for these patients despite their underlying disease, DM. According to the present report, the use of IFN-β1a was effective as a treatment option for COVID-19. Further clinical trial studies are recommended to examine the effect of treatment of IFN-β1a in patients with underlying disease or immunodeficiency.
AUTHOR CONTRIBUTIONS
MGJ and SM co-wrote the first and final drafts of this case report. HDC, MGJ, and SM all made significant contributions to the first major revision of the manuscript, final manuscript revisions, and approval of the final version.
ACKNOWLEDGMENTS
We are grateful to all participants who contributed to the collection and compilation of the present manuscript. All necessary ethical issues were observed, including observing fiduciary and respecting the patient's rights.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICAL APPROVAL
The Ethics Code for the study was IR.SSU.SRH.REC.1400.029 in Research Ethics Committee of Yazd Shahid Dr. Rahnemoun Hospital.
CONSENT
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Open Research
DATA AVAILABILITY STATEMENT
All data relating to this study are presented within the manuscript. Other materials are available from the corresponding author upon reasonable request.