17 alpha-hydroxylase deficiency: A case report of young Chinese woman with a rare gene mutation
Abstract
We report a young adult woman with 17 alpha-hydroxylase deficiency (17α-OHD) in Shandong province of China. The patient carried compound heterozygous mutations in the CYP17A1 gene: c.985–987 delinsAA (p.Tyr329LysfsX90) and c.1486C > T (p.Arg496Cys). The patient's hypertension and hypokalemia were resolved after taking medications of glucocorticoid, aldactone, and calcium antagonists.
1 INTRODUCTION
17 alpha-hydroxylase deficiency (17α-OHD) is a rare form of congenital adrenal hyperplasia (CAH) due to autosomal recessive disorders.1 The disease is caused by mutations in the gene of the cytochrome P450 family (CYP17) on chromosome 10.1, 2 The enzyme of 17 alpha-hydroxylase is essential for the synthesis of cortisol and adrenal gland hormones, and these hormones play a key role in the adrenal steroid production.3 The absence of the enzyme causes decreased cortisol levels and leads to high production of adrenocorticotropic hormone (ACTH) that further drives overproduction of 11-deoxycorticosterone (DOC) and corticosterone.1, 4 High DOC and corticosterone lead to hypertension, hypokalemia, and sexual infantilism and pubertal development failure.1, 5 The clinical and biochemical presentations can vary among patients with 17α-OHD. Therefore, genetic testing is crucial for the diagnosis of 17α-OHD.
In this paper, we reported the case of a female Chinese patient of 26–year-old who had 17α-OHD with clinical presentations of hypertension, hypokalemia, and female gonadal dysplasia, and with laboratory tests and genetic mutations that confirmed 17α-OHD.
2 CASE PRESENTATION
A 26-year-old Chinese woman was admitted to the Affiliated Weihai Second Municipal Hospital, Qingdao University, due to hypertension and hypokalemia, and symptoms of weakness. She firstly visited the reproductive clinic of the hospital three months prior to the admission because of hypertension (160/110 mm Hg) and hypokalemia. In her past history, she had not experienced menstruation before the age of 18 years. Later, she had irregular menstruation after a hormone replacement therapy (HRT) and returned to amenorrhea after stopping the HRT. She was married with no pregnancy, and her husband was healthy. Her parents were healthy and had normal pubertal and reproductive histories. She had two sisters, and her sisters were healthy and had normal pubertal development.
On physical examinations, she had female external genitalia, and immature breasts and vulva. Her breast reached at G2 stage (Tanner stage). She had no menstruation. Her blood pressure was 160/110 mm Hg. She was 160 cm tall with body weight of 55 kg and a body mass index (BMI) 21.48 kg/m2. Examination of the heart, lungs, thyroid, and abdomen revealed no abnormalities.
2.1 Biochemical and medical imaging tests
Ultrasound examination revealed that she had immature female ovaries or uterus. CT examination showed that she had right adrenal hyperplasia (Figure 1). The CT examination also found slightly left adrenal proliferation (Figure 1), but it was less obvious than the right side.
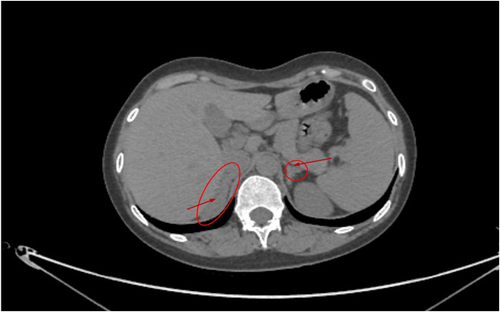
Laboratory investigations revealed normal hemoglobin and blood cell counts. Renal, liver, and thyroid function tests were normal, and the levels of calcium and phosphate were normal. The endocrine test results are presented in Table 1. The patient had a reduced level of potassium (3.2 mmol/L), estradiol (< 18.35 mmol/L), testosterone (< 0.087 mmol/L), and dehydroepiandrosterone sulfate (0.764 μmol/L), respectively, whereas her ACTH, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and aldosterone were quite high.
| Test items | Results | Reference range for female |
|---|---|---|
| Before the treatment | ||
| Potassium | 3.2 mmoL/L | 3.5–5.5 |
| Estradiol | < 18.35 mmoL/L | Follicular: 45.4–854; ovulatory: 151–1461; luteal: 81.9–1251; menopausal: 18.4–505 |
| Testosterone | < 0.087 mmoL/L | 0.29–1.67 |
| FSH | 15.64 mIU/ml | 5–10 |
| LH | 18.71 mIU/ml | 5–10 |
| Cortisol (8 am) | 92.96 nmoL/L | 133–537 |
| ACTH (8 am) | 81.56 pg/mL | 7.2–63.3 |
| 17-hydroxyprogesterone | 0.1 | < 0.1 |
| Dehydroepiandrosterone (DHEA)-S | 0.764 μmol/L | 2.68–9.23 |
| Aldosterone (laying) | 486.7 pg/ml | 30–160 |
| Aldosterone (standing) | 541.4 pg/ml | 70–300 |
| Plasma renin (laying) | 0.18 ng/ml/h | 0.15–2.33 |
| Plasma renin (standing) | 0.18 ng/ml/h | 0.10–6.56 |
| Chromosome karyotype | 46,XX | |
| CYP17A1 Mutations | c.985_987delinsAA (p.Tyr329LysfsX90), EX6; c.1486C > T(p.Arg496Cys), EX8 | |
| After the treatment | ||
| Potassium | 3.8 mmoL/L | 3.5–5.5 |
| Blood Pressure (BP) | 125/75 mmHg | < 140/90 |
- Abbreviations: ACTH, adrenocorticotropic hormone; FSH, follicle stimulating hormone; LH, luteinizing hormone.
2.2 Genetic testing results
The genetic testing result is showed in Table 1. The patient carried compound heterozygous mutations in the CYP17A1 gene: c.985–987 delinsAA (p.Tyr329LysfsX90) in exon 6, and c.1486C > T (p.Arg496Cys) in exon 8. The c.985–987 delinsAA mutation caused amino acid alterations with the tyrosine-329-Lysine (Tyr329Lysfs). The c.1486C > T mutation led to amino acid alterations with a substitution of arginine by cysteine (p.Arg496Cys). The patient's chromosome karyotype was 46,XX. The mutations are recorded as pathogenic according to the guideline of the American College of Medical Genetics and Genomics (ACMG).6 The c.985_987delinsAA mutation has been frequently detected in Chinese 17α-OHD patients.3, 7 However, the c.1486C > T (p.Arg496Cys) mutation has never been reported in Chinese 17α-OHD patients.
2.3 Treatments
During hospitalization, the patient received treatments to control high blood pressure and adjust serum electrolyte levels. The medications included dexamethasone (oral, dose of 0.75 g once a day), nifedipine (oral, 30 mg twice a day), and spironolactone (aldactone) (oral, 40 mg twice a day). Dexamethasone was started, and subsequently, nifedipine and spironolactone were given. The use of glucocorticoids was to normalize the blood levels of 11-DOC and ACTH and blood pressure. Nifedipine and spironolactone were administered in combination to control high blood pressure and normalize serum electrolyte levels, such as potassium level. After the treatment, her blood pressure had decreased to normal (125/75 mm Hg) and blood potassium level had increased to normal (3.8 mmoL/L) (Table 1). The patient was followed up in 2021, and her health status was stable with the maintained treatment.
3 DISCUSSION
Congenital adrenal hyperplasia is an autosomal recessive disorder due to a deficiency of one or more enzymes in the steroidogenesis process.1, 8 The most common CAH disorders are caused by 21-hydroxylase deficiency, accounting for approximately 90%–95% of all CAH cases,9 and the remaining 5%–8% of CAH cases are largely attributed to 11β-hydroxylase deficiency.10, 11 The 17 alpha-hydroxylase deficiency is a rare form of CAH, accounting for approximately 1% of CAH cases with an estimated incidence of approximately 1 in 1,000,000 newborns.3, 8 The first case of 17α-OHD was reported by Biglieri et al. in 1966, describing a 35-year-old female patient with hypertension, hypokalemia, and sexual infantilism.12 The first Chinese case was reported in 1982 by Shanghai Rui Jin Hospital.13 To date, there have been more than 500 cases of 17α-OHD reported worldwide,3 and more than 200 cases have been reported in China.14 To the best of our knowledge, 17α-OHD cases with the c.1486C > T (p.Arg496Cys) mutation in the CYP 17A1 gene have not been previously reported in China.
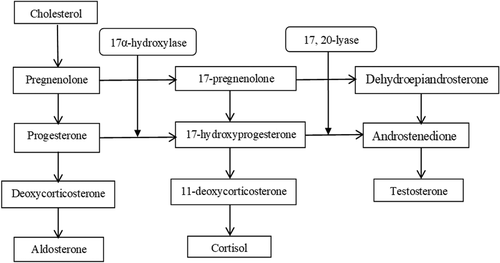
17α-OHD is due to mutations in the cytochrome P450c17, encoded by the CYP17A1 gene, located on chromosome 10q24-q25.1, 3, 11 The gene has eight exons and seven introns, spanning approximately 6.6 kb.15 In the steroid biosynthesis, the CYP17A1 enzyme catalyzes both steroid 17α-hydroxylase and 17, 20-lyase activities in the adrenal glands and gonads and plays a key role in cortisol and adrenal steroid production.5, 15, 16 Figure 2 shows that the 17α-hydroxylase catalyzes the transformation between pregnenolone and 17-pregnenolone, and between progesterone and 17-hydroxyprogesterone. The 17-pregnenolone and 17-hydroxyprogesterone are then collapsed by 17, 20-lyase and transformed into dehydroepiandrosterone (DHEA) and androstenedione, respectively. The lack of 17α-hydroxylase causes glucocorticoids and adrenal steroid deficiencies, which further stimulate the production of ACTH and 11-DOC as a compensatory process.1, 16 High levels of 11-DOC and ACTH cause hypopotassemia and hypertension. In the meantime, deficiency of 17α-hydroxylase leads to symptoms of sexual immaturity due to cortisol inadequacy and sexual hormone disorder.3
Most of the patients with 17α-OHD have symptoms of hypertension, hypokalemia, and sexual infantilism. Males with 17α-OHD often show pseudo hermaphroditism or female external genitalia due to androgen deficiency, and female patients have immature puberty development and primary amenorrhea due to a lack of estrogen during adolescence.17, 18 Common laboratory findings due to 17α-OHD include low levels of potassium and cortisol, and high ACTH, aldosterone, FSH, and LH. In the present report, the patient manifested symptoms are consistent with the common clinical presentations, and the biochemical analytical results are consistent with some other studies reporting a 17α-hydroxylase deficiency in adolescents and young adults.3, 15, 19, 20
Clinical presentation and biochemical manifestation are heterogeneous among patients with 17α-OHD due to the involvement of various kinds of hormones in the adrenal steroid production. It has been estimated that 10%–15% of cases have a normal blood pressure, and some patients experience mild hypokalemia or normal potassium level.3, 15, 20 Substantial variation in the severity of the disorder has been observed among 17α-OHD patients with the same mutation in the CYP17A1 gene. Previous studies suggest that there may be some environmental and other genetic factors than the CYP17A1 that could influence the clinical presentation or severity of the symptoms, such as hypertension.20 Therefore, the diagnosis of 17α-OHD should be based on overall evaluations of clinical, biochemical, and molecular characteristics. It is important to utilize genetic analysis for a diagnosis of 17α-OHD.
The prevalence of the gene mutations among 17α-OHD patients in China remains unclear. One study reported that among 181 Chinese cases with reported genetic mutations, 38.6% and 30.4% carried c.985_987delinsAA (p.Tyr329Lys) and c.1459_1467del (p.487_489del) mutations, respectively, and the remaining 31% had other mutations, while among non-Chinese patients, 92.8% of cases carried mutations other than c.985_987delinsAA (p.Tyr329Lys) and c.1459_1467 (p.487_489del).3 Another recent study reported a total of 68 Chinese patients with 17-OHD and found that 53.8% of the patients carried c.985_987delinsAA (p.Tyr329Lys) and 11.4% had c.1459_1467del (p. del D487_F489), which were the top two mutations among the examined cases.21 Previous studies suggest that genetic mutations among patients with 17α-OHD vary with race, and the c.985_987delinsAA mutation is common in the Asian population.22-24 In this study, the patient carried the c.985_987delinsAA mutation, which is consistent with previous studies showing that this mutation is prevalent in Chinese patients,3, 15, 22, 23 whereas the c.1486C > T mutation in this case seems rarely found in Chinese patients with 17α-OHD. The compound heterozygous variants of CYP17A1 may be the underlying causes of the 17-OHD in the patient as previous research has demonstrated that the c.985_987delinsAA and c.1486C > T mutations caused complete loss of 17α-hydroxylase activities and the associated hypertension, hypokalemia, and sexual infantilism.3, 25
Treatments for 17α-OHD mainly include glucocorticoid supplementation and adrenal steroid hormone replacement. Glucocorticoid helps to reduce 11-DOC and ACTH. Besides, calcium antagonist, spironolactone, angiotensin II receptor blockers, and cortisone are often used to control blood pressure. Steroid hormone replacement is to maintain female sexual characteristics.15 For patients with a karyotype of 46,XX, estrogen and progestin replacement therapy can be used to induce menstrual cycle. Before the implementation of treatments, physicians should discuss with patients or their parents for patients of children and adolescents about the effects and consequences of the treatments, and respect patients' selection of the treatments, such as gender preference. If a patient with a karyotype of 46,XY prefers to be considered male, androgen replacement may be considered. If the patient has cryptorchidism, a gonadectomy surgery may be performed as undescended intra-abdominal testes are subject to malignant deterioration.15 In this study, the patient's presented gender is in line with that in the chromosome karyotype (46,XX).
This case report has important implications for early diagnosis and effective interventions. Due to the low prevalence and the diverse clinical, biochemical, and molecular presentations, the early diagnosis of 17α-OHD is critical for early and proper interventions and treatments. Relative to other forms of CAH, the diagnosis of 17α-OHD is usually delayed to adolescence or adulthood due to previous absence of typical clinical symptoms and adrenal crisis.15, 18 Delayed diagnosis of 17α-OHD is particularly common in China due to challenges in receiving genetic testing or due to social or self-stigma for the disease. For the 17α-OHD patients, tests of plasma cortisol and ACTH can help to diagnose the disorder of 17α-OHD. In clinical practice, when adolescent or young adult patients present hypertension and hypokalemia, particularly when combined with primary amenorrhea and sexual infantilism, physicians should consider the possibility of the 17α-OHD disorder and recommend further evaluations for confirmation of the diagnosis. Molecular genetic tests could be helpful for the diagnosis of 17α-OHD patients.
4 CONCLUSION
In this paper, we reported a female patient with 17α-OHD who had c.985_987delinsAA and c.1486C > T mutations in the CYP17A1 gene, and major symptoms of hypertension, hypokalemia, primary amenorrhea, and sexual infantilism. Early diagnosis of 17α-OHD is important for early treatments and appropriate interventions to decrease the severity of the disorder and increase quality of life of the affected individuals. A combined analysis of clinical symptoms, biochemical examinations, and genetic testing should be considered in the diagnosis of 17α-OHD. Genetic testing is recommended to confirm the diagnosis when young patients have symptoms and biochemical indices that are suspicious for 17α-OHD. The interventions should be personalized and tailored to meet the needs of individual patients to achieve maximal health benefits in enhancing sexual development and fertility and reducing adverse consequences of 17α-OHD.
AUTHOR CONTRIBUTIONS
LHH conceived the study, analyzed, and interpreted the patient data and wrote the draft of the manuscript. LHH, LW, and XYW wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
The present case report was approved the Ethics Committee of the Affiliated Weihai Second Municipal Hospital of Qingdao University.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and the accompanying image.
Open Research
DATA AVAILABILITY STATEMENT
The datasets presented in this article are not readily available due to privacy and ethical restrictions.




