Native right-sided endocarditis in intravenous drug user: A case report
Abstract
An increased rate of hospitalizations due to right-sided infective endocarditis is currently witnessed due to the rapid rise of IV drug use. In this case report, we aim to discuss the long-term outcome and highlight the various diagnostic approaches and management difficulties that are encountered in these cases.
1 INTRODUCTION
Patients with infective endocarditis (IE) require extensive workup to ensure that timely, and effective management is provided and still poses a significant healthcare concern due to its high 1-year mortality of 30%.1 Right-sided IE poses a different challenge than left-sided IE due to its lower prevalence of about 10% and different etiological causes which are intravenous drug use (IVDU), intracardiac devices, central venous lines, and congenital heart diseases.1, 2 However, right-sided IE is thought to have more favorable outcomes as it usually affects young patients, is associated with fewer complications and less likely caused by drug-resistant microbes.3 An increased rate of hospitalizations due to right-sided IE is currently witnessed due to the rapid rise of IV drug use.
Intravenous drug use-associated IE prevalence has doubled from 2010 to 2015 with an estimated prevalence of 30% and is associated with a high mortality rate (8%).4, 5 Most common isolated microbes include Staphylococcus aureus (which is associated with worst clinical outcomes) and streptococcal species.5, 6 In this case report, we present a case of a 33-year-old female patient with a past medical history of polysubstance IV drug use and COVID-19 pneumonia that was presented to our emergency department (ED) in a lethargic state and later diagnosed with native tricuspid valve IE. We discuss the long-term outcome and highlight the various diagnostic approaches, need for multidisciplinary teams, and management difficulties that are encountered in such cases.
2 CASE PRESENTATION
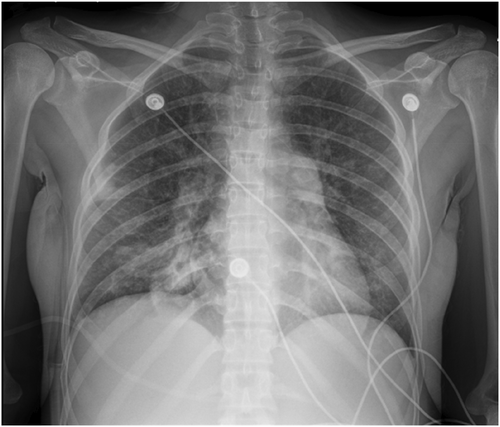
A 33-year-old female patient with a past medical history of polysubstance abuse and COVID-19 pneumonia presented to the ED with altered mental status, an obtunded state and only responsive to painful stimuli. The patients stated her last intake of methamphetamine was 2 days prior to admission. The patient was subsequently admitted to the critical care unit for further assessment and management. Chest X-ray (Figure 1) on admission showed bilateral patchy opacities which may indicate atypical/viral pneumonia or pulmonary edema. Computed tomography (CT) scan of the brain revealed no evidence of acute intracranial hemorrhage, midline shift, or mass effect.
Supportive therapy was initiated, and transthoracic echocardiogram (TTE), renal and bladder ultrasound (US), venous US of bilateral lower extremities, and a ventilation/perfusion (V/Q) scan (due to elevated D-Dimers) were ordered. Lorazepam was administered as needed for withdrawal symptoms. Empiric coverage with vancomycin and piperacillin, and tazobactam was started. Patient also received two units of packed blood red cells (PBRC).
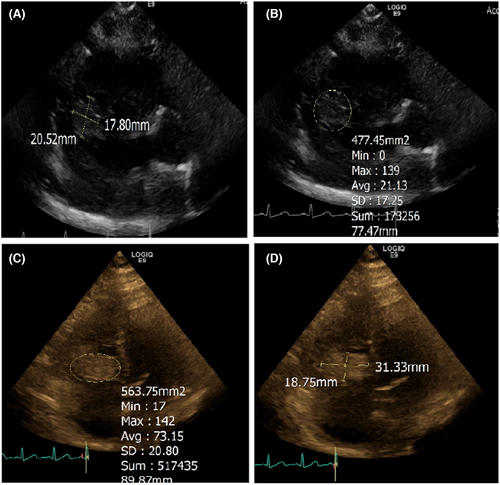
The TTE revealed thickening of tricuspid valve leaflets. There was no tricuspid valve stenosis. Moderate tricuspid regurgitation (TR), mild mitral regurgitation, and large tricuspid valve vegetation were present (Figure 2).
On 2nd day of admission, the patient developed fever (100.6°F) for which acetaminophen was administered. She also complained of left-sided sharp chest pain. Cardiac enzymes and electrocardiogram (EKG) showed no new findings. Her blood cultures, however, were positive for gram-positive cocci in clusters. The V/Q scan showed a high probability of bilateral pulmonary embolism. Additionally, the patient's stool was dark in color. Hemoglobin on Day 2 improved to 7.6 g/dl. She continued to have tachycardia; thus, medication was given for rate control. Target mean arterial pressure was >65 mmHg to maintain adequate cerebral perfusion.
On the 3rd day of admission, the patient's urine output was low, blood urea nitrogen (BUN) was 102 mg/dl, creatinine was 2.95 mg/dl while sodium level was 127 mEq/L. The patient was started on sevelamer for hyperphosphatemia.
On the 4th day of admission, her hemoglobin dropped from 7.3 to 6.9 g/dl; she was tachypneic and exhibited labored breathing. Repeat chest X-ray showed worsening infiltrates and right-sided effusions concerning developing heart failure. CT chest was also done which revealed peripheral pulmonary nodules consistent with septic pulmonary emboli. Multiple cavities, wedge-shaped consolidations, air bronchograms, and feeding vessel sign (findings consistent with septic pulmonary emboli). There appeared to be a pulmonary embolus in the left posterior basal segment, though evaluation was limited on the non-contrast CT scan. Pulmonary trunk was slightly enlarged. Mild-to-moderate pleural effusion on the right side was observed with mediastinal lymphadenopathy, hepatomegaly, mild splenomegaly, and small volume ascites. Blood cultures were positive for S. aureus susceptible to oxacillin. Therefore, 1 g of oxacillin was started every 12 h instead of vancomycin. The patient continued to remain oliguric, and her renal function continued to worsen; therefore, dialysis was started.
On the 6th day of admission, the patient continued to have elevated white blood cell (WBC) count of 17.9 × 109 cells per liter and fever documented at 103.3°F. Chest X-ray revealed mild interval worsening of bilateral hazy and consolidative patchy opacities. Right lung appeared worse than the left lung. These opacities may represent atypical/multifocal pneumonia. There was also worsening of pleural effusion on the right side.
On the 10th day of admission, her blood pressure (BP) fluctuated throughout the day, and she was tachycardiac at 120 s. She received a transfusion of PRBC. Hemodialysis was continued for her oliguria. A repeat TTE ordered to evaluate for any possible left-sided heart involvement was done which was negative. The cardiothoracic surgeon recommended angiovac for vegetation debulking.
On 16th day as plans for her possible transfer to another facility for the cardiac procedure were being discussed, her laboratories revealed very low C3 and C4 levels. An antinuclear antibody comprehensive panel was ordered for possible Lupus. However, the laboratories came back negative. A repeat TTE showed that the vegetation had decreased in size. The vegetation now measured approximately 3.34 × 1.26 cm which suggested reduction in size from the previous size of 3.62 × 1.6 cm on admission.
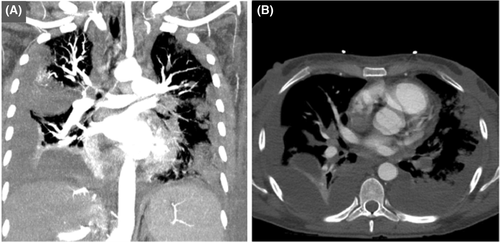
On 18th day of admission, the patient remained tachycardic. A V/Q scan was repeated which showed a high probability for multifocal bilateral pulmonary emboli. When compared to the scan done on admission, the defect in the left lower lung appeared larger. CT angiography (Figure 3) of the chest revealed a large occlusive left-sided pulmonary embolism at the level of the left interlobar artery with evidence of right heart strain. Additional smaller segmental and subsegmental pulmonary emboli in the right middle and bilateral upper lobes were also seen. There was redemonstration of multifocal nodular opacities, some with cavitation suspicious for septic emboli. There were increased subpleural consolidations in the left lung, and in the setting of large pulmonary embolism, it could not exclude pulmonary infarct. There were moderate-to-large bilateral pleural effusions, right greater than left.
A TTE done on the same day showed normal left ventricular wall thickness, mildly hypokinetic mid-anteroseptal segment, left ventricular ejection fraction of 45–50%. Right ventricle was mildly to moderately dilated. Severe TR was observed, and the tricuspid vegetation now measured 2.5 × 1.9 cm. On 22nd day of admission, the patient's BP was low, with tachycardia. Her WBC count continued to increase, and she remained febrile through the day. One dose of tobramycin was given, and she was started on micafungin. On Day 24, due to worsening pleural effusion, thoracentesis was performed and 700 cc of serosanguinous fluid was removed from the pleural cavity and sent for culture and cytology. At the time of finalizing this manuscript, the patient was awaiting to be accepted to a hospital for Angiovac and vegetation debulking.
3 DISCUSSION
This case report describes a challenging case of right-sided IE and its complications owing to a history of polysubstance IVDU. Infective endocarditis is by far one of the most dreaded complications of IVDU. IE is diagnosed using the modified Duke Criteria scoring system.3 A high index of suspicion is often required for diagnosis, especially in IE caused by S. aureus as these patients are less likely to present with systemic findings.7 Our patient's diagnosis was definite for right-sided IE as she fulfilled two major criteria (positive blood culture with S. aureus from two separate blood cultures and TTE evidence confirming endocardial involvement) and three minor criteria (history of injection drug use, fever, and vascular phenomenon in the form of multiple pulmonary emboli).3
Infective endocarditis is well established to cause a variety of immunological phenomena which includes Osler nodes, Roth spots, and immune complex-mediated glomerulonephritis.3 Another manifestation could be low complement levels as was seen in our patient. Low complement levels in patients with IE are usually associated with glomerulonephritis and worsening of renal function.8 However, in our case the acute renal injury was pre-renal in origin due to the low FENA and elevated BUN to creatinine ratio.
Intravenous antibiotics are the most vital intervention to treat right-sided IE affecting the tricuspid valve.1 Guidelines indicate that antibiotic treatment should be started promptly, with empiric treatment at first with amoxicillin and gentamicin if the presentation is indolent or vancomycin and gentamicin if there are signs of sepsis.9 As per the guidelines, vancomycin was immediately started in our patient with addition to anti-pseudomonal antibiotics since the patient's history of IVDU puts her at an increased risk for Pseudomonas infection. Antibiotic therapy was later changed to oxacillin guided by the results of her cultures as indicated by the guidelines. Duration of therapy is recommended at 4–6 weeks10; however, recent guidelines suggest TTE should be used to guide treatment in patients that suffer from complications as in our case.7
Certain cases, however, do not improve with medical management and warrant surgical intervention. The guidelines indicate that clinical features like heart failure, uncontrolled infection, and large vegetation size warrant cardiac surgery.7 Other indications include septic pulmonary emboli and persistent bacteremia despite medical therapy.1 A recent study that spanned over a period of 10 years concluded that surgery conferred no long-term survival advantage in people with IE due to IVDU due to a high rate of reinfection.6 The timing for surgical intervention also plays an important role in IE management to ensure the best possible outcomes. Guidelines suggest waiting 1–2 weeks after antibiotic therapy before pursuing cardiac surgery for left-sided IE, however, early surgery is warranted in patients with neurological complications, large vegetation (>15 mm), recurrent emboli, and uncontrolled infection.11 In this case report, surgical intervention was warranted at around the 2nd week of admission but it was delayed due to difficulties in admitting the patient to a hospital that could perform the surgery.
Interestingly, our patient presented with right-sided IE following a COVID-19 pneumonia infection. Several cases in the literature describe development of IE following COVID-19 pneumonia, which may be a risk factor to this patient's severe infection course. In a case report by Alizadeh et al.,12 the authors have discussed a case of a 50-year-old male patient who tested positive for COVID-19 and had to be intubated due to hemodynamic instability. The patient tested positive for S. aureus bacteremia, and his echocardiogram revealed evidence of vegetation in the aortic valve area. IE in this patient led to development of acute ischemia and extensive thrombosis, and the patient had to undergo an elective forefoot amputation and made good recovery.12 In another study by Ouarradi et al.,13 a clinical timeline of two patients was discussed, both of whom first tested positive for COVID-19, followed by resolution of respiratory symptoms but ended up developing fever and dyspnea after a few days, to be diagnosed with IE as seen in our patient. One of the patients developed shock and multivessel failure, and the other developed neurological complications, resulting in death in both the cases.13 Finally, Blogova et al.14 concluded in their study on post-COVID endocarditis that SARS-Cov-2 infection induces non-bacterial thrombo-endocarditis or infective endocarditis, autoimmune mechanisms playing a significant role in both these cases. Persistence of coronavirus in the myocardium for a long time can also be considered as a contributing factor toward endocarditis.14
4 CONCLUSION
There is a need for timely diagnosis, with a high index of suspicion in intravenous drug users, and management by multidisciplinary teams for better outcome in patients with Staphylococcus-associated IE. Considerations for surgical repair should be discussed with cardiothoracic surgeons and weighed against the chances of reinfection and long-term benefits. More awareness is required regarding the population abusing IV drugs, and clinics that would promote prevention by reaching out to these individuals and providing help.
AUTHOR CONTRIBUTIONS
HF, AM, FC, AI, and MR has written the manuscript. NBP and VJ performed critical edits of the draft and prepared the final version of this manuscript which was approved by all authors.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
CONSENT
Written informed consent has been taken from the patient for this case.
Open Research
DATA AVAILABILITY STATEMENT
Available upon reasonable request from Corresponding Author.




