Fulminant hepatitis following COVID-19 vaccination: A case report
Abstract
The common side effects of COVID-19 vaccination were mostly self-restricted local reactions that quickly resolved. Nevertheless, rare autoimmune hepatitis cases have been reported in some vaccinated with mRNA COVID-19 vaccines. This article presents a young man who developed fulminant hepatitis a few days after vaccination with the first dose of the AstraZeneca COVID-19 vaccine. A 35-year-old man was admitted to our hospital with generalized weakness, abdominal pain, and jaundice. He received the first dose of the AstraZeneca COVID-19 vaccine 8 days earlier. He was admitted to the hospital with a chief complaint of abdominal pain. On admission and because of his high D-dimers, low platelet count, and low Fibrinogen level, vaccine-induced immune thrombosis thrombocytopenia was suspected, which was ruled out later. Then, after a surge in his liver function tests, decreasing platelet, and abnormal clotting tests, fulminant hepatitis was considered for this patient. Several bacterial, viral, and autoimmune etiologies were then suspected, with all ruled out. Thus, fulminant hepatitis secondary to his AstraZeneca COVID-19 vaccine was confirmed. Unfortunately, he died 3 days later of disseminated intravascular coagulopathy, after which a liver necropsy was performed, indicating drug/toxin-induced hepatitis.
1 INTRODUCTION
The common side effects of COVID-19 vaccination were mostly self-restricted local reactions that quickly resolved.1 Nevertheless, rare autoimmune hepatitis cases have been reported in some vaccinated with mRNA COVID-19 vaccines.2, 3 This article presents a young man who developed fulminant hepatitis a few days after vaccination with the first dose of the AstraZeneca COVID-19 vaccine.
2 CASE PRESENTATION
A 35-year-old man was admitted to our hospital with generalized weakness, abdominal pain, and jaundice. He received the first dose of the AstraZeneca COVID-19 vaccine 8 days earlier. Five days after vaccination, the patient experienced fever and headache. Despite the improvement of fever, his abdominal pain was exacerbated, and loss of appetite, jaundice, and vomiting was also manifested. Thus, he was admitted to our center. He had a history of psychological problems under control with paroxetine and sodium valproate. His laboratory results on admission are summarized in Table 1. According to the high D-dimers, low platelet count, and low fibrinogen level, vaccine-induced immune thrombosis thrombocytopenia was suspected. Therefore, high-dose dexamethasone 40 daily, IVIG 1 mg/kg for 2 days, and rivaroxaban 15 mg daily were started for the patient. Abdominal ultrasonography showed a grade I fatty liver disease, normal gall bladder, and pancreas with mild effusion in subdiaphragmatic space. Also, no intra- or extra-hepatic biliary dilatation was observed. Color Doppler ultrasonography revealed normal portal and hepatic vein and inferior vena cava. The laboratory results of his second hospitalization day were indicative of a surge in his hepatic enzymes, decreasing platelet, prolonged partial thromboplastin time, and increased international normalized ratio (Table 1) (Figure 1A).
| Characteristic | Reference value | Findings | |
|---|---|---|---|
| On admission | During hospitalization | ||
| Age (year) | 35 | ||
| Sex | Male | ||
| Preexisting conditions | Psychological problems | ||
| Time from vaccination to admission (day) | 8 | ||
| Symptoms and signs | Severe abdominal pain, loss of appetite, jaundice, icteric sclera, and vomiting | ||
| Platelet count (per μl) | 150,000–400,000 | 50,000 | 27,000 |
| d-dimer (ng/ml) | <500 | 15,000 | >18,000 |
| Fibrinogen (mg/dl) | 200–400 | 179 | 153 |
| INR | 1 | 1.5 | |
| PTT (s) | 25–45 | 40 | 51 |
| LDH (U/L) | <480 | 4800 | >5400 |
| CRP (mg/L) | <10 | 66 | 86 |
| ESR (mm/h) | <20 | 3 | 5 |
| Bilirubin total (mg/dl) | <1.2 | 4.7 | 15.3 |
| Bilirubin direct (mg/dl) | <0.4 | 1.5 | 3.7 |
| AST (U/L) | 5–40 | 1000 | 4700 |
| ALT (U/L) | 10–55 | 2000 | 5900 |
| ALP (U/L) | 24–147 | 461 | 713 |
- Abbreviations: ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; INR, International normalized ratio; LDH, Lactate dehydrogenase; PTT, Partial thromboplastin time.
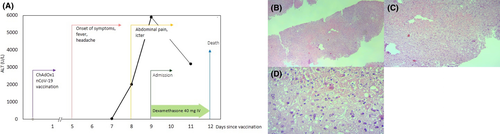
Furthermore, laboratory results were negative for hepatitis A, B, C, and E viruses, HIV, EBV, HSV-1, and HSV-2. Anti–nuclear antibody, anti–smooth muscle antibody, and SARS-CoV-2 reverse transcriptase-polymerase chain reaction were also negative. Hence, fulminant hepatitis was suspected in the patient. Due to a lack of clinical improvement, he became a liver transplant candidate. Unfortunately, after 3 days of hospitalization, he expired as of disseminated intravascular coagulation, after which a liver necropsy was performed, indicating drug/toxin-induced hepatitis (Figure 1B–D).
3 DISCUSSION
Vaccines have been shown to trigger an immune response leading to a broad spectrum of autoimmune diseases.4 The spike glycoprotein of SARS-CoV-2 or adenoviral vector spike protein vaccines share genetic similarities with a large heptapeptide human protein, so this is an additional factor that can trigger autoimmune disease after vaccination due to molecular mimicry.5 In our patient viral markers were negative, and there was no history of drug or toxin exposure. Unfortunately, our patient died quickly after hospitalization, making our laboratory tests incomplete in determining the etiology of his fulminant hepatitis. On the other hand, the patient was being treated with paroxetine and sodium valproate for his psychological illnesses that might have increased the chance of fulminant hepatitis following vaccination. Since these side effects are infrequent, such cases should not discourage people from being vaccinated against COVID-19, yet physicians must be vigilant for such potential adverse events.
AUTHOR CONTRIBUTION
The case was diagnosed and followed up by NR and MB. SE and MB conceived and planned the case report, MB, ASR, and TTS wrote the manuscript, and MB and TTS edited the first draft and provided substantial revision. The final version was read, corrected, and approved by all authors. All co-authors take full responsibility for the integrity of the case study and literature review.
ACKNOWLEDGEMENTS
The authors thank the Department of Infectious Diseases and Tropical Medicine of Babol University of Medical Sciences.
CONFLICT OF INTEREST
TTS reports that he provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc. and also as a member of the Advisory Board to Galera Therapeutics, which are not in any way associated with the content or disease site as presented in this manuscript. All other authors have no relevant financial interests to be declared.
CONSENT
Written informed consent was obtained from the patient for publication of the current case report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.