Diagnostic evaluation and assessment of anemia in a patient with chronic kidney disease and gastrointestinal angioectasias undergoing hemodialysis
Abstract
Anemia in patients with chronic kidney disease may have underlying causes that require a broad approach. Here, we present a clinical case of anemia in a patient with chronic kidney disease and gastrointestinal angioectasias undergoing hemodialysis.
1 INTRODUCTION
Management of anemia in chronic kidney disease (CKD) patients in hemodialysis must be tailored. Keeping hemoglobin levels within adequate ranges depends on nutritional, inflammatory, mechanical, and immunological factors unique for each patient and on their response to erythropoiesis-stimulating agents. Hence, each patient requires a personalized approach and treatment.
2 CASE PRESENTATION
This is a 68-year-old male patient with a long history of type 2 diabetes mellitus (20 years) treated with low dose intermediate insulin, hypertension controlled with calcium channel blockers and angiotensin-converting enzyme inhibitors (ACE inhibitors), pulmonary emphysema secondary to smoking, and stage 5 chronic kidney disease, receiving hemodialysis three times per week since 2017. In February 2018, he received an arteriovenous dialysis graft for vascular access. He was hospitalized in June 2018 to treat an ulcer in his left foot associated with his underlying diabetes, which required debridement and specialized wound care, with good evolution and discharge after 10 days with an oral antiplatelet agent.
In August 2018, he presents a sudden episode of pallor and weakness at home, and he is taken to the Emergency Department, where he is hospitalized with a diagnosis of lower gastrointestinal (GI) tract bleeding, with digital rectal examination revealing hemorrhoids and blood-stained feces. His first laboratory results showed hemoglobin at 4.8 g/dl (2.98 mmol/L) and a mean corpuscular volume (MCV) of 95 fl. His hemoglobin 1 month prior was 12.3 g/dl (7.63 mmol/L), so he received 3 units of packed red blood cells (PRBC) and a gastroenterologist is brought in for a consultation. During his hospitalization, he remained stable, without new bleeding episodes and he was discharged with hemoglobin at 8.9 g/dl (5.52 mmol/L), with an appointment for a colonoscopy and antiplatelet suspension. He continued his hemodialysis as an outpatient without heparin, increasing his erythropoietin (Epo) beta to 10,000 intravenous (IV) Units (U) (400 U/kg·week) every hemodialysis session.
In September 2018, 2 weeks after he was discharged, he was taken to the Emergency Department with a new episode of haematochezia and hemoglobin at 7.0 g/dl (4.34 mmol/L), MCV at 92 fl, and a red blood cell distribution width (RDW) below 2. An urgent colonoscopy was performed, finding two angioectasias (angiodysplasias) in the ascending colon, without any other findings in the mucosa. Unfortunately, angioectasias were left untreated because, at the time of colonoscopy, thermo-coagulation with either argon plasma or heat probe options was not available at the hospital. He received one unit of PRBC and was scheduled for future thermo-coagulation of the angioectasias once argon plasma was functional.
His hemoglobin levels were monitored monthly, as well as platelets and coagulation studies remain normal. The patient received PRBCs as needed until thermo-coagulation could be performed in April 2019. Colonoscopy was performed, and four vascular malformations, compatible with angioectasias, were observed in the ascending colon (Figure 1) and treated with argon plasma thermo-coagulation, without complications.

He continued with IV Iron supplements during hemodialysis and Epo, though beta Epo had to be changed to alpha Epo at an equivalent dose, because of unavailability at that time.
During follow-up of his anemia, an upper endoscopy was done in August 2019 that showed only gastric erosions. Since he remained with hemoglobin levels below 10 g/dl, and had episodes of scant but active bleeding in the caecum, a video capsule endoscopy was ordered. In September 2019, he underwent another colonoscopy for follow-up, but no evidence of angioectasias was found; only a single isolated diverticulum in the ascending colon with the rest of the mucosa reported as normal. Hematology was then consulted for assessment, since there was no evidence of major gastrointestinal bleeding and the patient persisted with anemia. Bone marrow and peripheral blood analyses were conducted to complete the diagnostic assessment.
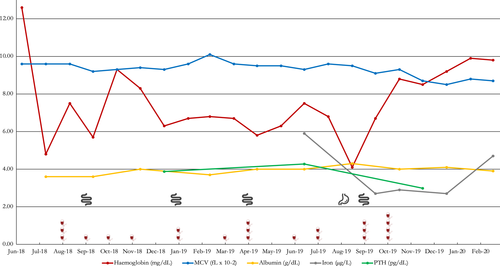
Figure 2 presents a graphical depiction of the case timeline and the transfusions and endoscopies conducted and the hemoglobin response.
 ) and upper (
) and upper ( ) and lower (
) and lower ( ) endoscopies conducted and the haemoglobin response
) endoscopies conducted and the haemoglobin response3 INVESTIGATIONS
We collected as much information related to the multiple causes of anemia to work through the differential diagnosis. Several laboratory studies are used in evaluation in hemodialysis patients to adjust the use of hematopoietic agents. The patient hematology work up included a bone marrow aspiration that showed normocellular content, medullar hyperplasia, and no evidence of parvovirus B19. Iron kinetics showed normal low iron levels (59 mcg/dl), low saturation (18%), normal folic acid and B12 levels. Parathyroid hormone (PTH) values remain in 385 pg/mL average and protein electrophoresis ruled out multiple myeloma. With the aim of identifying lesions in the gastrointestinal tract, the patient also underwent various endoscopic procedures (Figure 2).
To define the causes of persistent anemia in this patient, it is key to keep target hemoglobin values proposed in treatment guidelines.1 In this particular case, it was difficult to raise hemoglobin values above 9.0 g/dl (5.59 mmol/L) with transfusions only (Figure 2), since his blood type was O negative and it was difficult to find compatible units to transfuse. Our hospital has only one gastroenterologist, which also limits the possibilities to schedule follow-up appointments and procedures in a short period of time.
4 DIFFERENTIAL DIAGNOSIS
Anemia in CKD hemodialysis patients is multifactorial, and the variability in the values of hemoglobin depends on blood losses (e.g., gastrointestinal bleeding or coagulation in the hemodialysis system), infections (e.g., catheter infections or diabetic foot), chronic disease inflammatory processes (e.g., diabetic foot, hyperparathyroidism, malignancies), nutritional deficit (e.g., decreased levels of iron and cofactors, such as B12 vitamin and folic acid), metabolic causes (e.g., hypo- or hyperthyroidism), certain drugs (e.g., angiotensin-converting enzyme inhibitors), and frequent changes in plasma volume due to accumulation of interdialytic liquids.2
Active gastrointestinal bleeding must be ruled out: Hemodialysis patients may present gastrointestinal anemia, with lesions in the gastric or duodenal mucosa and between 4% and 13% may present with angioectasias.3 In about 45% of the cases, there may be a co-infection with Helicobacter pylori,4 and as a predisposing factor to bleeding, with a mucosal lesion after exposure to anticoagulants three times per week.
Among hematological causes of anemia, multiple myeloma must be ruled out, looking for abnormal proliferation of plasma cells, with plasmocytes in the bone marrow and production of monoclonal proteins.5 Pure secondary red blood cell aplasia presents as a normocytic, normochromic, sudden, progressive, and severe anemia mediated by antibodies. It is characterized by complete absence of the red blood cell precursors in the bone marrow and may be due to malignancies, immune or viral causes, or secondary to certain drugs, such as Epo.6 In some patients, resistance to Epo may be present and causes, such as hyperparathyroidism, nutritional deficits, infections, and drug–drug interactions, must be explored in order to correct it.1
5 TREATMENT
In this case, angioectasias were successfully treated with thermo-coagulation with argon plasma. Studies show that for angioectasia-related gastrointestinal bleeding, therapeutic options include endoscopic therapy (i.e., laser photoablation, thermo-coagulation with argon plasma, and bi- or multipolar electrocoagulation), certain drugs as octeotride (not available at that time), thalidomide or conjugated estrogen; and surgery.7, 8 Angioectasias locations were key determining treatment effectiveness.
While target hemoglobin established in the guidelines for treating anemia is not achieved, patients must receive transfusions to keep hemoglobin level until the cause of bleeding is found and treated, thus, decreasing morbidity and mortality associated with cardiovascular causes.6
Although there was no evidence of new gastrointestinal bleeding or any other treatable cause was identified, the patient remained with anemia, and with a low Epo responsiveness. Since no antibodies to red blood cells were found, peripheral smear showed no abnormalities of morphology, and direct coombs test was negative, the colleagues at The Hematology Department recommended long-acting Epo, metoxipolyethylenglycol epoietin beta Continuous Erythropoieisis Receptor Activator (CERA), at 200 μg every 15 days since December 2019 and supplementation of parenteral iron, with dose adjustments when hemoglobin reached a value of 10 g/dl.
6 OUTCOME AND FOLLOW-UP
The patient stabilized his hemoglobin, without need for any more transfusions after December 2019. At 6 months, he presented with mean hemoglobin levels of 11.0 g/dl (6.83 mmol/L) and remains without decrease of hemoglobin to the date of this report. To authorize this case report, the patient signed an informed consent as well as consent to publish the data. The Institutional Committee for Ethics in Research at the Caja de Seguro Social approved this case report, No. CIEI-CSS-M-071-2020 PVS-014-2020, dated 07 July 2020.
7 DISCUSSION
We report a clinical case of a 68-year-old male with a long history of type 2 diabetes mellitus, hypertension, pulmonary emphysema, and stage 5 chronic kidney disease treated with hemodialysis who presented with severe anemia with acute worsening secondary to angioectasias. Angioectasias are vascular malformations with a frequency of 19% to 32% much higher in CKD hemodialysis patients than in normal kidney function patients. The prevalence is considered as correlated to the duration and severity of the chronic kidney disease.9 The development of these lesions may be secondary to intermittent low-grade obstruction of submucosal veins associated with an increased proliferation depending on the vascular endothelial growth factor, hypo-oxygenation of intestinal mucosa, the use some drugs, as non-steroidal anti-inflammatory drugs (NSAIDS), antiplatelet drugs, and sevelamer phosphate binder.10 Several studies suggest that patients that acquired angioectasias have risk factors, as peripheral vascular disease, chronic constipation, diabetic neuropathy, advance age, that in time will produce intermittent blood flow to the intestinal mucosa, that contributes to a degenerative vascular process that ends in hemorrhagic episodes, most commonly observed in the intestinal mucosa in the terminal ileon and the colon.11
In about 90% of the cases, bleeding is self-limited, but in the remaining 10%, endoscopic, surgical, or medical treatment is required. The most common treatment methods are non-contact endoscopic techniques, such as thermo-coagulation with argon plasma.12 Angioectasias in CKD hemodialysis patients may present with chronic anemia, resistant to Epo, or with recurring digestive bleeding that will require frequent transfusions.13, 14 During the time the patient was followed up by gastroenterology and receiving transfusions, he kept normal levels of parathyroid hormone (PTH), with normal phosphorus values, so he did not have a phosphate binder among his medication.
About 5% to 10% of patients using erythropoiesis-stimulating agents do not respond as expected, that is, an increase of 1.0 to 1.5 g/dl (0.62 to 0.93 mmol/L) on the total hemoglobin during the correction phase. In this case, the patient started with Epo beta, then was switched to Epo alpha and finally received long-acting Epo to stabilize his hemoglobin levels. The dose of Epo depends on each patient's requirement and will vary according to weight, response, and the presence of any factor that may drive resistance. The long-acting product may allow dose reductions once serum iron deposits go up to normal values.15
Of note, the erythropoiesis response to stimulating agents will not necessarily increase with a higher dose. When this is the case, it is important to look for other causes of anemia and to avoid the use of doses in excess of the recommended therapeutic doses of Epo to prevent dose-dependent side effects, such as hypertension and cardiovascular complications due to an increased blood viscosity, including but not limited to occlusion of vascular access.
Concentrations of c-reactive protein above 20 mg/L (190.5 nmol/L) have been associated with Epo resistance, and some studies have associated it with an increase of up to 80% of the dose of erythropoiesis-stimulating agents. The patient c-reactive protein values were below 10 mg/L (95.2 nmol/L).
This case is of interest because it should remind us that anemia in patients receiving hemodialysis may become a diagnostic challenge, as it is multifactorial and will require participation of diverse clinical specialties and a well-orchestrated treatment.
8 CONCLUSIONS
- Anemia in patients with chronic kidney disease receiving hemodialysis is multifactorial, and all possible causes must be ruled out.
- Gastrointestinal bleeding in hemodialysis patients due to angioectasias is more prevalent that in normal renal function patients.
- Angioectasias in chronic kidney disease in hemodialysis can occur as hemorrhagic episodes but can also persist as an occult bleeding.
- Since factors contributing to chronic kidney disease also contribute to angioectasia, it is important to keep in mind all different treatment approaches to provide an early an appropriate management.
- The use of blood transfusions has decreased since the discovery of erythropoiesis-stimulating agents, but blood transfusions are still important in the management of anemia.
AUTHOR CONTRIBUTIONS
IL involved in conceptualization the study, interpreting data, and writing the manuscript. WB, DQ, MP, and NB involved in interpretation of the clinical data. KC involved in conceptualization the study, collecting clinical data, and writing the manuscript.
ACKNOWLEDGMENTS
The authors would like to recognize the work of Humberto López Castillo, MD, PhD, for his support reviewing the manuscript and preparing the images. Iván Landires is member of the Sistema Nacional de Investigación (SNI), supported by the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Panama.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ETHICAL APPROVAL
The manuscript was approved by institutional review boards or local ethics committee.
CONSENT
Written informed consent was obtained from the patient to report the present case.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




