Presentation and treatment of aggressive, Triple-Negative carcinosarcoma of the breast
Abstract
An extremely rare form of breast cancer, breast carcinosarcoma accounts for less than a percent of all breast malignancies and is highly aggressive. Composed of both cancerous epithelial and mesenchymal cell types, breast carcinosarcoma is associated with a poor prognosis compared to more common breast cancers, and typically lack the receptors typical of other breast carcinomas, which minimize potential targets for treatment. In this case report, we discuss a 56-year-old patient affected by carcinosarcoma of the breast at a T2N1 stage, and the decision-making process that factored into her treatment plan.
1 INTRODUCTION
A rare and aggressive tumor, carcinosarcoma is a mixed, dedifferentiated carcinoma consisting of an epithelial, carcinomatous component and a non-epithelial, mesenchymal component.1 Distinct from other metaplastic carcinomas, carcinosarcoma is crucially biphasic2-4 lacking a clearly identifiable transition zone5 and historically has been known by several names, including biphasic metaplastic carcinoma, sarcomatoid carcinoma, and metaplastic sarcomatoid carcinoma.6
Various organs have been known to be affected by this malignancy, including the skin, breast, bladder, ovaries, lung,1 larynx,7 and the uterus,8 where uterine carcinosarcomas are known as malignant mixed Müllerian tumors.9 A diagnosis of carcinosarcoma in the breast typically accompanies a poor prognosis because of the tumor's propensity toward lymph, pleural, and pulmonary metastasis.1
Evidence suggests that the hybrid components of breast carcinosarcoma have a monoclonal origin10 from an original breast myoepithelial (or its precursor) cell that differentiate in a biphasic manner.5, 11A proteomics study by Djomehri et al. suggests that relative to non-metaplastic breast carcinomas, breast carcinosarcoma is upregulated in its epithelial–mesenchymal transition pathway and is downregulated in its oxidative phosphorylation pathway.12
In the breast, carcinosarcoma accounts for less than 0.2–1% of new cases.5, 13-15 One oft-cited study of this malignancy demonstrated a cumulative 5-year survival rate of 49%, with a precipitous decrease in survival odds at later stages of the disease. Lacking a treatment standard,5 options for remedying breast carcinosarcoma include surgical resection, chemotherapy, and radiation,1, 16 although recurrence of disease has been known to occur in these approaches toward treatment.2
2 CASE REPORT
56-year-old woman presented to the hospital with a palpable mass 12 cm from the nipple. An ultrasound revealed a neoplasm in the upper inner quadrant of the left breast, with a diameter of 2.2 cm (Figure 1A). Having developed within the span of a year, initial impressions of the mass were unremarkable and thought to be benign. EKG results for the patient were similarly unremarkable. The patient reported an unconfirmed medical history of untreated ovarian and cervical cancers, as well as fibroids.
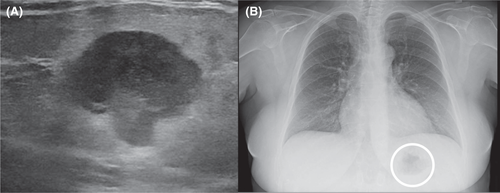
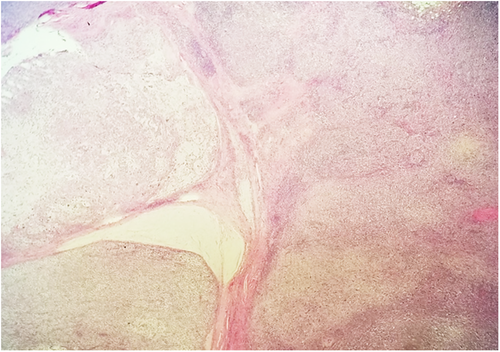
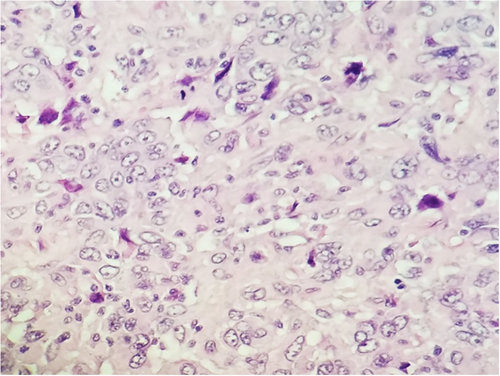
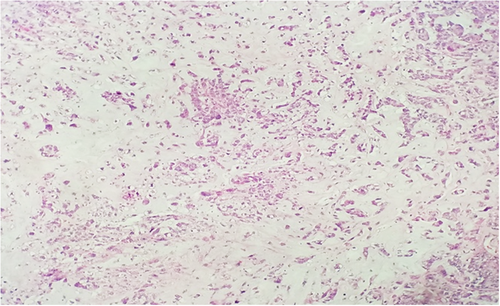
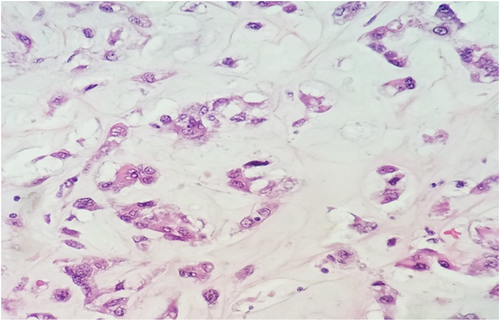
Subsequent biopsy and immunohistochemical staining on the breast mass indicated it was a malignancy negative for ER, PR, and HER2, though positive for E-cadherin and p-120 catenin. Following a chest X-ray (Figure 1B), as well as a lumpectomy of the mass and surrounding tissue, the malignancy was diagnosed as a ductal-type carcinoma, with troubling sarcomatous, squamous, and chondroid elements (Figures 2-5). The tumor was an irregularly shaped, white-tan mass of ductal origin with dimensions of 3.0 × 2.3 × 1.7 cm that had become partly necrotic and adjoined to an 8 mm diameter cyst. While the skin overlying the tumor and the marginal tissue surrounding the tumor were benign, positive vimentin staining confirmed that elements of the malignancy were of a mesenchymal origin. Other immunohistochemistry results indicated positive stains of AE-1, AE-3, GATA-3, P63, PD-L1, and s100, but negative stains of SMA, actin, desmin, factor XIIIa, CD34, CD68, and CD117. The cancer was shown to have greatly metastasized into the left axillary lymph node. The tumor was highly proliferative, with a Ki-67 expression of 80%.
The patient, who has a medical history of anemia, was found to have depleted erythrocyte and hemoglobin levels at the time of treatment, as well as elevated platelet counts. The patient's alkaline phosphatase levels were elevated, which suggested the tumor may have metastasized into the skeletal system and caused lesions. This possibility was ruled out following PET/CT and bone scans.
3 DISCUSSION
The carcinosarcoma exhibited by the patient falls into a larger umbrella category of metaplastic breast carcinomas (MpBCs),15 a rare type of neoplasm accounting for 0.5%–3% of mammary carcinomas.17 According to WHO guidelines, MpBCs are classified as spindle cell, squamous cell, matrix-producing, carcinosarcoma, or osteoblastic,18 although they can be further described according to unique histotype.5, 15, 17, 19 A biphasic MpBC, carcinosarcoma contains both epithelial and sarcomatous components,15 indicated by co-expression of epithelial indicators like AE-1/3, and mesenchymal indicators like vimentin and SMA.17 Differential histological diagnoses of MpBC include myoepithelial carcinoma, myofibroblastic tumors, malignant phyllodes tumors, pleomorphic adenoma, and adenomyoepithelioma.20
Poorly understood and difficult to diagnose and treat, MpBC poses a greater risk than more common types of breast cancer. MpBC was not officially recognized as a distinct histological carcinoma until 2000,21 which has limited research on this devastating cancer.17, 22 Breast carcinosarcoma resembles the clinical features of invasive ductal carcinoma1 and is often treated in a similar way, utilizing anthracycline and taxane-based regiments.11 This treatment paradigm, however, contradicts growing evidence that MpBC is a distinct molecular entity.23 MpBCs have a high incidence of a triple-negative (TN) phenotype,15 lacking receptors for estrogen, progesterone, and HER2,12, 23-25 limiting targeted treatment options.11 One retrospective analysis by Xuexin et al. concluded that in TN MpBC patients, chemotherapy was not associated with improved survival.26 Large-scale studies have investigated how receptor presence impacts the survival outcomes of MpBC patients, although the results are contradictory. A study by Schroeder et al. found that HER2 positivity was correlated with greater MpBC survival than other MpBC molecular subtypes,27 but a similar study by Jinquian et al. did not find this effect.28 A study by Hu et al. compared patients with TN MpBCs to patients with receptor-positive MpBCs, and found that only the patients with the TN molecular subtype experienced greater survival rates after radiotherapy.29 Other researchers have found that patients with TN MpBC phenotypes have worse prognostic outcomes than non-TN MpBC patients.26, 29 In case-matched population analyses of TN MpBCs, TN MpBC patients have a more aggressive disease course and worse prognostic outcomes compared to TN nonmetaplastic breast cancer patients.13, 25, 26, 30-32
The patient's breast neoplasm stained positively for GATA3, an intriguing finding considering the neoplasm's negative estrogen receptor (ER) status. GATA3, an ER-linked transcription factor,33-35 stains in high association with estrogen receptor expression.36 In studies that stain for GATA3 across distinct ER-positive mammary carcinomas, GATA3 staining reaches positivity rates between 60%–100%.34, 37 As MpBCs tend to have TN histotypes,23 the utility of GATA3 staining is mixed. Studies into the topic are often hampered by low sample sizes, but a review on the topic found that GATA3 stained ER-negative metaplastic breast carcinomas at rates between 17% and 56%, depending on the study.34 In light of these difficulties, Di et al. proposed the transcription factor TRPS1 as a novel biomarker for TN MpBCs, being expressed in 86% of cases relative to GATA3's 21%.38
Genetic analysis of the patient's breast mass indicated mutations in CDH1, raising alarm. This gene encodes E-cadherin, a transmembrane glycoprotein that maintains cell–cell adhesion.39 Germline mutations in CDH1 predispose individuals to hereditary diffuse gastric cancer,40 and when these mutations are detected, patients undergo prophylactic gastrectomies.41, 42 The patient's germ cells were genotyped, from which a CDH1 mutation was ruled out. No further procedure was necessary.
It is important to note that the tumor's initial diagnosis based on biopsy results alone suggested the mass was a ductal and lobular carcinoma, while further examination of the excised tumor revealed an identity of carcinosarcoma. This underscores the importance of proper needle placement in the biopsy site, as it is evident that diagnosing the malignancy based on tumor component alone is difficult to validate, as other studies have noted.11
The patient's tumor presented treatment challenges. Typical of other breast carcinosarcomas,3, 5 it was a triple-negative malignancy at risk of visceral metastasis earlier rather than later in the course of disease. Because such tumors accompany poorer prognoses than receptor-positive tumors,43 the patient began an aggressive course of adjuvant chemotherapy, as well as a course of radiation at the lumpectomy site. Two years into treatment, the patient achieved remission with no sign of relapse.
Clinical studies on breast carcinosarcoma indicate that after surgery, chemotherapy combined with radiation therapy are correlated with better survival outcomes than that of either treatment alone. In one case of triple-negative breast carcinosarcoma in a 49-year-old, radiotherapy dosed at 50 Gy provided in conjunction with anthracyclines and taxanes successfully treated the disease, with no evidence of relapse in the fourth year of follow-up.44 A PR-positive case of carcinosarcoma involving a 34-year-old patient utilized a similar treatment course and had similarly positive results 5 years after surgery. One study matched 24 patients with metaplastic breast carcinomas to patients with non-metaplastic breast cancers of the same TNM stage, and found that with aggressive early treatment, MpBC patients can achieve comparable survival outcomes to patients with more typical breast carcinomas.11
4 CONCLUSION
This case report describes the presentation and treatment plan of carcinosarcoma of the breast. A rare disease, carcinosarcoma of the breast is difficult to treat and as such, clinical study and reporting of its treatment is invaluable for improving patient outcomes.
AUTHOR CONTRIBUTIONS
NV reviewed the literature and patient information and wrote and revised the manuscript. TOJ and LE edited the manuscript. MS provided pathology images. VKG edited the manuscript and organized the project. All approved the final manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare. No funding was provided for this submission.
CONSENT
Patient consent has been signed and collected in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




