Pure white cell aplasia an exceptional condition in the immunological conundrum of thymomas: Responses to immunosuppression and literature review
Abstract
Thymomas are tumours frequently associated with autoimmune manifestations or immunodeficiencies like Good syndrome. In rare cases, pure white cells aplasia (PWCA) has been described in association with thymomas. PWCA is characterized by agranulocytosis of autoimmune background primary refractory to granulocyte colony-stimulating factor (G-CSF). It is necessary the use of immunosuppressor to allow granulocyte recovery. Without treatment, it could be fatal.
1 INTRODUCTION
PWCA is a hematologic disorder characterized by agranulocytosis with absence of myeloid precursors in bone marrow with an erythropoiesis and megakaryopoiesis preserved. It has been associated with drugs, infectious diseases and autoinmunity.1 Thymomas and thymic carcinomas may present autoimmune phenomena fundamentally: myasthenia gravis (MG) up to 50% of the cases and pure red cell aplasia (PRCA) up to 5% of the cases.2 However, the incidence of thymoma and PWCA is extremely rare by existing few reports.3
We conducted a search for articles registered in Pub-Med between 1950 and 2021, which were available in English. The keywords used were as follows: Thymoma, Pure White Cell Aplasia, Agranulocytosis, and Granulocytopenia.
2 CASE
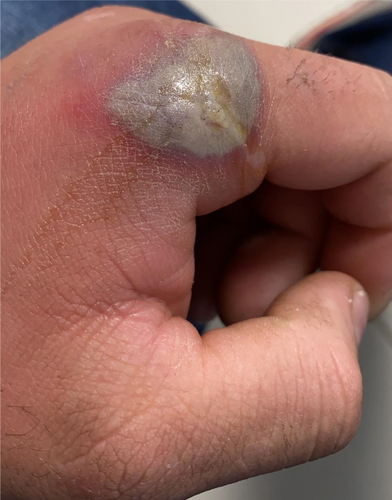
A 33-year-old male, with a history of admission to the intensive care unit for influenza A in 2016, consulted for skin lesions at primary care and was given treatment with amoxicillin/clavulanic acid and ibuprofen in October 2020. Three days later, he consulted the emergency department of our hospital due to the worsening of clinical symptoms with thermometric tympanic fever of 40.5°, blood pressure 123/67 mmHgm, and heart rate 98 beats per minute. He had not taken any other medication or drugs previously. Examination revealed a 2 cm branching ulcer on the oral mucosa and four indurated skin lesions with an erythematous halo and necrotic center, suggestive of gangrenous ecthyma (Figure 1), the largest on the left hand measuring 3 cm in diameter.
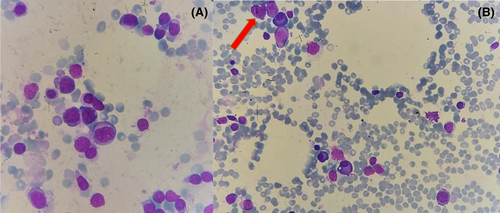
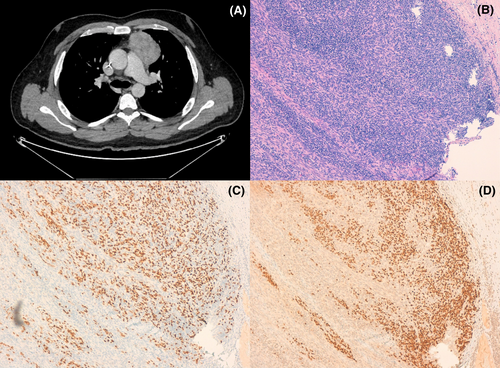
Laboratory tests on admission showed normal renal, liver, and thyroid function, C-reactive protein 3104.7 nmol/L (Normal Range 0–1100), procalcitonin 1400 ng/L (NR 0–100), hemoglobin 139 g/L (NR 130–160), platelets 161 × 109/L (NR 150–450), and leukocytes 0.5 × 109/L (NR 4.5–10) (Revised formula: 100% lymphocytes). IgG 4.79 g/L (NR 6–17), IgA 0.57 g/L (NR 0.7–4) IgM 0.69 g/L (NR 0.4–2.30), C3 and C4 normal. Anti-nuclear, anti-neutrophil (ANCA), anti-musk, and anti-acetylcholine antibodies were negative. Serology for HBV, HCV, and HIV were negative; he tested IgG + against EBV and CMV. Peripheral blood flow cytometry analysis showed CD4+/CD8+ ratio inversion 0.56, low B lymphocytes, but no data suggestive of B/T clonality. Blood cultures, nasal swab for S. aureus as well as bacterial culture and PCR of skin lesions were negative. Bone marrow aspirate showed normal erythroid and megakaryocytic series. Granulocytic series represented 3.6% of the total cellularity, promyelocyte maturational arrest was observed (Figure 2). There was no evidence of dysplasia or increased blast cellularity, karyotype 46, XY. CT scan revealed an anterior mediastinal mass measuring 47 × 71 × 60 mm with no evidence of locoregional infiltration. A biopsy of the mass was performed with an anatomopathological result of mixed type AB thymoma (Figure 3).
On admission, empirical antibiotic therapy was started with piperacillin/tazobactam and daptomycin with the improvement of symptoms and resolution of fever in the following days. With the initial diagnosis of agranulocytosis, G-CSF 480 mcr/24 h was added to the treatment for 13 days with no increase in the neutrophil count, so it was discontinued. Once the diagnosis of thymoma was confirmed and with the suspicion of related PWCA, single dose of intravenous Immunoglobulin G (IVIG) 1 g/Kg, and ciclosporine (CyA) with target levels 200–300 ng/dl were initiated. On Day +10, there were signs of granulocytic recovery: Neutrophils 0.17 × 109/L in peripheral blood; so, G-CSF was associated; on Day +14 from the start of CyA the patient reached neutrophils 17 × 109/L. Thymectomy was performed on Day +21, without remarkable incidents.
After thymectomy, CyA tapering was started. Six months later CyA was discontinued, neutrophil count remains still stable seven months after discontinuation: leukocytes 7.5 × 109/L, neutrophils 4 × 109/L, hemoglobin 148 g/L, and platelets 185 × 109/L. Immunoglobulin levels, CD4+/CD8+ ratio and B lymphocytes returned to normal values. The patient has not presented any infectious, CyA-related or post-surgical complications during follow-up.
3 DISCUSSION
Immunity may be impaired in patients with thymoma. Thymoma-associated immunodeficiency is known as Good's syndrome and includes hypogammaglobulinemia, decreased or absent B lymphocytes, CD4+/CD8+ inversion, and decreased T lymphocytes. In addition, autoimmune manifestations may occur.4 The etiology of thymoma related PWCA is still unknown, but it appears to have an autoimmune background. Growth inhibition of granulocytic and macrophage colony-forming units exposed to different concentrations of serum from these patients has been observed. This finding suggests the presence of an immunoglobulin against immature myeloid cells, indicating an alteration of B cells and humoral immunity.5 Conversely, the response to anti-calcineurin immunosuppressors in these cases, as in PRCA, points to an alteration in T cells and cellular immunity.6 Thymus is the organ where T-cell maturation and TCR gene rearrangement occurs. Besides, it is the place where negative selection of autoreactive T cells and positive selection of T cells capable of recognizing MHC presented antigens take place.7 In this sense, several causal mechanisms for the loss of self-tolerance in thymoma patients have been proposed: (1) Immaturity of neoplastic T cells that would allow the escape of autoreactive lymphocytes, (2) Neoplastic genetic alterations that would predispose to the appearance of autoimmunity such as decreased expression of HLA-DR, and (3) Theory of combined dysregulation of cellular and humoral immunity, an autoreactive T cell would activate a B cell to produce autoantibodies.8
Surgery to resect tumor tissue is the standard treatment for patients with thymoma. Thymectomy could resolve the autoimmune manifestations by removing the neoplastic tissue, which seems to provide the antigenic stimulus for autoreactive cells.
It has been reported the case of a patient with thymoma and granulocytopenia in whom a decrease in anti-pANCA antibody titer and elevation of granulocytes in peripheral blood was observed after thymectomy.9 However, in other cases neutropenia has not resolved after thymectomy and a second line of treatment is necessary.10, 11 The medical treatment of these patients is not established currently due to low incidence of cases. Several strategies have been used to increase granulocyte counts (Table 1). GCS-F and IVIG normally have no impact in granulocytic count.12 Of the 24 patients collected 13 survive, all of them receive some immunosuppressive treatment (CyA 6 patients, azathioprine 2, corticoid 2, alemtuzumab 1, chemotherapy 1, and plasmapheresis 1) which reinforces the idea of a combined surgical and immunosuppressive treatment for these patients.
| CASE | REFERENCE | AGE | SEX | THYMOMA HISTOLOGY | BONE MARROW | DEBUT AND ALTERATIONS | 1ºLINE THERAPY (DAYS UNTIL RESPONSE) | RELAPSE | 2º (DAYS UNTIL RESPONSE) | SERUM INHIBITOR | EXITUS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Josse JH, 1958 | 73 | F | Spindle cell | Hypoplasic y amegacarocytosis | Fever and caquexia | Antibiotics (R) | – | – | – | Yes |
| 2 | Thiele H G, 1967 | 53 | M | Spindle cell | Promyelocyte arrest | ND | – | – | – | – | Yes |
| 3 | Rogers BHJ, 1968 | 69 | F | Spindle cell | Hypoplasic | Anemia, trombocytopenia bleeding and petechial Hypogammaglobulinaemia | Prednisone +Testosterone (R) | – | Thymectomy (R) | – | Yes |
| 4 | Jacobson BM, 1971 | 70 | F | Spindle cell | Promyelocyte arrest | Fever Hypogammaglobulinaemia ANA+ | Splenectomy (R) | – | Prednisone +Isoniacide (R) | – | Yes |
| 5 | Young RH, 1977 | 68 | F | Spindle cell | Myelopoiesis absent | Fever Hipogammaglobulinemia Reumatoid Factor+ | – | – | – | – | Yes |
| 6 | Degos L, 1982 | 52 | F | Spindle cell | Promyelocyte arrest | Recurrent infections Hypogammaglobulinaemia and absent B lymphocytes | Thymectomy Prednisone and Cyclophosphamide (R) | – | Plasmapheresis (ND) | Yes | No |
| 7 | Ackland SP, 1988 | 70 | F | Metastasis malignant Spindle cell | Myelopoiesis absent | Pulmonary sepsis Hypogammaglobulinaemia Miastenia gravis | IVIG (R) | – | – | Yes | Yes |
| 8 | Weir AB, 1989 | 64 | M | Spindle cell | Promyelocyte arrest | Fever Hypogammaglobulinaemia CLL | Vincristine +prednisone (6) | Yes | Vincristine +prednisone (14 days) Thymectomy (6 days) | Yes | Yes |
| 9 | Nagashima S, 1989 | 58 | M | Spindle cell | Promyelocyte arrest | Reordenamiento TCR β? Anti-AChR | Radiation (R) | – | Prednisone, thymectomy (?) | No | No |
| 10 | Mathieson PW, 1990 | 46 | F | Lymphoepitelial | Promyelocyte arrest | Mucotutaneus ulcers Hypogammaglobulinaemia Miastenia gravis | Plasmapheresis (R) | – | Azatioprine +prednisone (120 days) | Yes | No |
| 11 | Postiglione K, 1995 | 68 | F | Spindle cell | Promyelocyte arrest | Trombosis ANA+ | G-CSF +IVIG + Prednisone (R) | – | Thymectomy, Plasmapheresis +Cyclophosphamide (7) | Yes | Yes |
| 12 | Yip D, 1996 | 51 | M | Spindle cell | Promyelocyte arrest | Fever Hypogammaglobulinaemia Anti-MUSK | Prednisone, CHOP and thymectomy (R) | – | G-CSF (6 days) G-CSF maintenance | No | No |
| 13 | Yip D, 1996 | 52 | F | Spindle cell | Myelopoiesis absent | Fever, mucocutaneus ulcers ANA+ | G-CSF, prednisone (R) | – | IVIG, cyclophosphamide (R) | – | Yes |
| 14 | Crawford WW, 1999 | 59 | M | Spindle cell | Myelopoiesis nearly absent | Diarrhea, dysphagia Hypogammaglobulinaemia CD4:CD8 inversion and low B lymphocytes | Methylprednisolona, and Azathioprine (21) NO Thymectomy performed | – | – | – | No |
| 15 | Fumeux Z, 2003 | 76 | F | Cortical B2 | Myelopoiesis absent | Fever and weight lose | Thymectomy +IVIG+ GCSF +Metilprendnisolone pulse (7) | Yes | CyA, Metilprednisolone +G-CSF (3) | – | No |
| 16 | Alvares CL, 2004 | 59 | M | Spindle cell | Myelopoiesis absent | Fever and mucocutaneus ulcers Anti-AChR Hypocomplementemia | G-CSF (R) Plasmaphersis (R) | Yes | Alemtuzumab (12) Alemtuzumab +CyA + MMF +GCSF | Yes | No |
| 17 | Jethava Y, 2011 | 45 | M | AB thymoma | Myelopoiesis absent | Fever and sepsis Hypogammaglobulinaemia XI Factor deficiency | CyA +Thymectomy (10) | Yes | CyA (7) | – | No |
| 18 | Akinosoglou K, 2014 | 70 | F | Spindle cell | Promyelocyte arrest | Absent B lymphocytes Low IgA and IgM Cryptococcal infection | Dexamethasone +G-CSF +IVIG (20) | – | – | – | No |
| 19 | Okusu T, 2016 | 72 | M | B2 thymoma | Granulocytic hypoplasia | Candida albicans | – | – | – | – | Yes |
| 20 | Olivera M, 2018 | 66 | F | AB thymoma | Hypoplasia and Promyelocyte arrest, displastic | Recurrent infection Hypogammaglobulinaemia absent B lymphocytes | Thymectomy, IVIG and G-CSF (R) | – | CyA (>30 days) | – | No |
| 21 | Kobayashi Y, 2018 | 63 | M | Spindle cell | Myelopoiesis absent | Fever Hypogammaglobulinaemia | G-CSF (R) | – | CyA (10) | Yes | No |
| 22 | Uy K, 2019 | 65 | F | Mixed AB2 | Promyelocyte arrest | Fever and rash Hypogammaglobulinaemia | Thymectomy (R) | – | CyA +G-CSF (7) | – | No |
| 23 | Case | 33 | M | Mixed AB | Promyelocyte arrest | Fever, mucocutaneus sepsis Hypogammaglobulinaemia CD4:CD8 inversion | G-CSF (R) | – | CyA +IVIG (10) | – | No |
- Abbreviations: Anti-AChR, anti-acetilcholine antibody; Anti-MUSK, anti-smooth muscle antibody; CLL, Chronic Lymphocytic Leukemia; CyA, cyclosporine A; M, male; F, female; G-CSF, Granulocyte colony-stimulating factor; IVIG, intravenous inmunoglobuline G; MMF, micophenolate mofetil; ND, no data; R, refractory; TCR, T-Cell receptor.
CyA has demonstrated favorable responses in these patients. It has been used with target trough levels of 200–400 ng/mL and monitoring toxicities. Granulocytic recovery occurs within 7–10 days. Maintenance treatment has usually been applied, with CyA tapering until its total suspension after 4–6 months.5, 10 Others have used extended treatment with CyA and prednisone in decreasing doses for up to 20 months after thymectomy.12
Alemtuzumab has been successfully used as an immunosuppressor in autoimmune bone marrow failures. In two cases of PWCA, alemtuzumab has achieved complete response in the first month.13 Alemtuzumab has been useful in the treatment of a patient with PWCA and thymoma, after the failure of G-CSF and plasmapheresis, achieving granulocyte recovery in 12 days. However, agranulocytosis relapsed 5 months later and was treated with a new cycle of alemtuzumab associated with CyA and maintenance mycophenolate.14
A case has been described of a patient with MG thymectomized, who relapsed after 12 years with MG and de novo PWCA. In this case, plasmapheresis was started with the improvement of the MG symptoms as diagnosis, but there was no change in the granulocyte count after 15 sessions. Azathioprine 2.5 mg and prednisone mg/kg were started, obtaining an increase in the granulocyte count 4 months later.15 It suggests that plasmapheresis alone is not a good option for the treatment of PWCA, and the use of concomitant immunosuppressor is needed.
Thymectomy is a major surgery with high complexity and infectious risks. We consider that the appropriate management would be the resolution of the PWCA prior to surgery. According to our review, treatments with immunosuppressive drugs are associated with better outcome. In our patient, we have obtained a good response with CyA, which supports the existing literature as the most successful therapeutic option. Furthermore, it is a drug with a known safety profile, extensive experience in its use and the possibility of measuring levels. Therefore, we suggest the use of CyA as a first-line drug with the concomitant use of G-CSF from granulocyte recovery onwards. Long-term follow-up of thymoma and immunological status is advisable because relapses have been observed in these patients.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
The authors report no conflicts of interest associated with this publication.
AUTHOR CONTRIBUTIONS
Roberto Céspedes López is the main author, was involved in patient care, provided necessary data for the article, and manuscript preparation. Elena Amutio Díez is the main reviewer, was involved in manuscript preparation, checked grammatical and data errors, and patient care. Xabier Martín Martitegui was involved in patient care and provided data for the article. Amaia Balerdi Malcorra was involved in patient care. Lucía Insunza Oleaga was involved in patient care and provided data for the article. Javier Arzuaga Méndez was involved in patient care and provided data for the article. Maite Moreno Gámiz was involved in patient care and provided data for the article. Mónica Sainz Camín provided pathology images and their pathological description. Yoseba Aberasturi Plata provided pathology images and their pathological description. Juan Carlos García-Ruíz checked the manuscript for grammatical and scientific errors.
CONSENT
Appropriate informed consent was taken for the publication of this report and the associated images. The authors have confirmed that the patient consent has been signed and collected in accordance with the journal consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.