The landscape of investigator-initiated oncology trials conducted in mainland China during the past decade (2010–2019)
Ye Cao, Lin-Miao Ye, and Zhong Fan contributed equally to this study and shared the first authorship.
Abstract
The number of clinical trials conducted in mainland China, including investigator-initiated trials (IITs), has increased rapidly in recent years. However, there are few data on the characteristics of cancer-related IITs. We performed a comprehensive analysis of the landscape of cancer-related IITs in mainland China in the past decade. All cancer-related IITs registered on two clinical trial registries in the United States (www.clinicaltrials.gov, CT.gov) and mainland China (www.chictr.org.cn, ChiCTR) from 2010 to 2019 were identified. IITs were reviewed manually to validate classification, subcategorized by cancer type, and stratified by design characteristics to facilitate comparison across cancer types and with other specialties. A total of 8199 cancer-related IITs were identified. The number of trials registered annually increased over time, especially in the last 5 years. Although interventional studies were predominant, randomized double-blind studies accounted for only 8% of IITs. In the past decade, the trend for interventional studies conducted with different drugs increased year on year, although the increase in hormonal therapy IITs was not significant. Additionally, cancer-related IITs were unevenly geographically distributed, with half concentrated in the economically developed cities Shanghai, Beijing, and Guangdong. We also found an increase in registration before participant enrollment (64.9% for trials in conducted in 2015–2019 vs. 40.2% in 2010–2014, p < 0.001) and data monitoring committee use (44.5% vs. 40.0%, p = 0.001) and a decrease in randomization (51.5% vs. 62.7%, p < 0.001) and funding (36.4% vs. 56.3%, p < 0.001) between these periods. We also observed changes in intervention type (decrease in cytotoxic drug therapy [34.8% vs. 48.9%, p < 0.001]; increase in targeted therapy [17.8% vs. 14.2%, p = 0.004], immune checkpoint inhibitor therapy [6.6% vs. 0.0%, p < 0.001], and immune cell therapy [9.6% vs. 4.5%, p < 0.001]). Details of cancer-related IITs conducted during the past decade illustrate the merits of oncology research in mainland China. Although the increased quantity of IITs is encouraging, limitations remain regarding the quality of clinical trials, regional imbalances, and funding allocation.
Abbreviations
-
- ChiCTR
-
- Chinese Clinical Trial Registry
-
- CT.gov
-
- ClinicalTrials.gov
-
- IITs
-
- investigator-initiated trials
-
- IQR
-
- interquartile range
-
- MeSH
-
- medical subject headings
-
- Multi
-
- multitumor classification
1 INTRODUCTION
With continuous developments in economy and standard of living, the epidemiology of human disease has changed rapidly in recent years. According to current estimates, malignant tumors will become the global leading cause of death from noncommunicable disease in the 21st century. The most recent cancer statistics data estimate that there were 19.3 million newly diagnosed cancer cases and 10 million deaths globally in 2020, among which 23.7% of newly diagnosed patients and 30.2% of deaths came from China, ranking first among all countries [1]. Moreover, with an increasingly aging population, the cancer burden in China will continue to rise, and the number of new cases and deaths is expected to reach 6.85 million and 5.07 million, respectively, by 2040 [2]. To reduce this burden, the Healthy China Action Plan (2019–2030) was established to strengthen basic and clinical research on cancer prevention and treatment, and to improve the overall level of cancer science and technology in mainland China [3]. It is, therefore, necessary to evaluate the current landscape of cancer treatment in mainland China to understand the existing strengths and weaknesses, guide policy-making, and facilitate future improvements.
Clinical trials are an important source of definitive evidence to guide medical decision-making, and can be conducted by industry or by clinical investigators. The latter are generally academic researchers with no direct commercial objective [4, 5], and the trials they conduct are routinely referred to as investigator-initiated trials (IITs). Compared with industry-initiated clinical trials, IITs are typically characterized by diverse study objectives, novel study designs, and greater alignment with clinical diagnosis and treatment needs, and may thus be better positioned to promote the depth and breadth of cancer-related research and play a key role in promoting the development of drugs and other treatment strategies, especially for rare malignancies. In recent years, IITs have received increasing attention in Chinese academia and national government agencies, and have also gained recognition in the international academic community (e.g., the C-TASK FORCE trial) [6].
To our knowledge, current published studies on the landscape of cancer-related clinical research in China are primarily focused on industry-initiated trials. As an example, the progress of China's registered anticancer drug clinical trials from 2008 to 2019 was reported by Li et al., and outlined trends in anticancer drug development in China for the first time [7]. In addition, most publications on the characteristics of clinical trials focus on specific tumor types (e.g., prostate cancer, non-small cell lung cancer) or specific treatments (e.g., cytotoxic therapy) [8-10]; few studies have focused on IITs, with only Japanese investigators reporting the characteristics of IITs in Japan from 2012 to 2014 [11]. No in-depth analysis of the current status of cancer-related IITs in mainland China has been reported to date; thus, the current study was conducted to address this knowledge gap.
2 METHODS
2.1 Search strategy and selection criteria
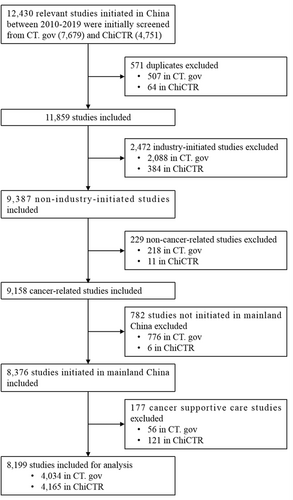
We searched ClinicalTrials.gov (www.clinicaltrials.gov, CT.gov) and the Chinese Clinical Trial Registry (www.chictr.org.cn, ChiCTR; the main location for registration of Chinese studies) for cancer-related studies registered in mainland China from January 1, 2010 to December 31, 2019. The medical subject headings (MeSH) terms “cancer” OR “tumor” OR “neoplasm” OR “carcinoma” OR “leukemia” OR “lymphoma” were used for the preliminary screening. The location was restricted to mainland China. In this study, IIT was defined as a clinical study initiated by an investigator at a hospital, university, or research institute; thus, studies were included if the applicant's institution in ChiCTR or the responsible party in CT.gov was a hospital, university, or research institute. We excluded duplicates, industry-initiated studies, noncancer-related studies, and studies initiated by sites not on the Chinese mainland. Studies related to cancer supportive care, defined as the best care for cancer pain, cancer-related fatigue, and various adverse effects (e.g., vomiting, leukopenia) experienced during chemotherapy, were also excluded based on the primary endpoint. The final list of cancer-related IITs in the mainland China were included in the analysis.
2.2 Data extraction and assessment
Two of the authors (YC and LM) independently searched CT.gov and ChiCTR with the same search algorithm to confirm the identified studies on April 4, 2020. Two other authors (WJ and DY) extracted the studies from the two registries. Deduplication was performed, both as intra-database deduplication (i.e., deduplication of studies with partially identical characteristics in the same database) and interdatabase deduplication (i.e., deduplication of the same study registered in both databases). Two authors (LM and LY) screened the duplicates in each of the registries according to the registry ID. Studies in both CT.gov and ChiCTR were deduplicated if they were identical in terms of title, leading site, investigator, and enrollment. Studies initiated by industry as well as studies not initiated in the mainland China, were excluded by two authors (LM and LY) according to study sponsor and location. Two authors (WY and LM) assessed the data to determine the cancer type for each study as well as to identify noncancer-related studies, and cancer supportive care studies were also removed at this stage. The above screening and assessments were performed independently by two authors. Next, 24 oncological specialists (volunteers) were divided into three groups to identify detailed interventional measures, including drug type (including cell immunotherapy). One author (YC) was consulted in the case of disagreement.
Tumor types were classified into primary classification by MeSH subject word (C04.588), and specific cancer types (e.g., lung cancer, breast cancer) were classified as the secondary classification. Where the disease could not be classified according to the specified primary classification (e.g., sarcoma and melanoma) or could be classified under a different primary classification according to inclusion criteria, the designation “Others” was used for the primary classification. Less frequently studied cancer types (fewer than five studies) under the primary classification were also classified as Others, and studies of diseases that could be included as multiple cancer types under the same primary classification based on inclusion criteria were classified into the multi-tumor classification (multi) (Supporting Information: Table S1).
Interventional studies involving drugs were classified into four categories according to drug category: cytotoxic therapy, targeted therapy, hormone therapy, and immune checkpoint inhibitor therapy. Further subdivision was not conducted owing to the scarcity and relative complexity of other types of drug. In addition, it was found that some studies classified cellular immunotherapy as a type of drug therapy, so cellular immunotherapy was included in this analysis.
2.3 Data synthesis
Owing to the pattern heterogeneity of CT.gov and ChiCTR, we reassigned the study category (e.g., drug, procedure, radiation), funding type, responsible party, masking, and randomization to ensure common pattern homogeneity. Given that the majority of multicenter studies in both databases had missing site information, the geographic location where studies initiated was identified based on the primary sponsor's address in ChiCTR and the responsible party's address in CT.gov. Indicators such as study type (e.g., interventional, observational) and study design were not reassigned because the original definition and/or study concept differed between the CT.gov and ChiCTR databases. For example, the concept of observational study was quite different between these two registries.
2.4 Statistical analysis
Data processing and statistical analyses were conducted using Excel 365 (Microsoft Corporation). All variables were assessed in a descriptive manner, providing number and percentage for qualitative variables and ranges, medians, and interquartile range (IQR) for quantitative variables. A simple regression model was used to analyze 10-year trends in the number of initiated IITs and the number of drugs. A simple stratification analysis was performed for specific indicators (e.g., drug type, endpoint distribution) by tumor type and study type.
3 RESULTS
3.1 Results of the search strategy
We retrieved a total of 12,430 cancer-related clinical studies that initially met our search requirements in CT.gov and ChiCTR. A total of 8199 cancer-related IITs from medical or scientific study institutions in the mainland China from 2010 to 2019 were subsequently included in our analysis after verifying the inclusion and exclusion criteria (Figure 1).
3.2 Timing trends of initiated IITs
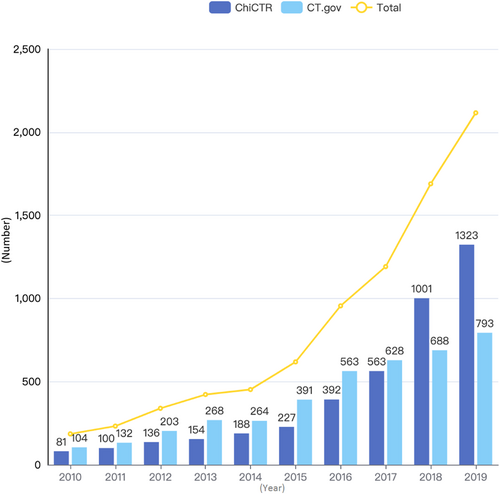
The number of cancer-related IIT registrations has increased significantly over time (see Supporting Information: Videos S1 and S2), with an average annual increase of 31.8% (Figure 2). Studies in 2018 and 2019 accounted for 46.4% (3805/8199) of the total, of which the number in 2019 was 11.4 times (2116/185) that in 2010. Registrations on ChiCTR have surpassed those on CT.gov since 2018, with a 45.5% increase (313/688) in 2018 compared with CT.gov and a significant increase of 77.8% from the previous year in 2018 alone.
The primary classification of cancer-related IITs included 11 major categories, with pediatric tumors and gynecological tumors as two independent categories (Figure 3a). Of the IITs in which cancer classification was identified, digestive-tract tumors were most commonly studied (38.9%, 3193/8199), followed by thoracic tumors (15.8%, 1296/8199) and hematological tumors (10.9%, 891/8199).
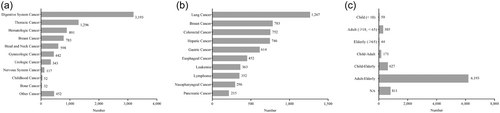
The top 10 cancer types in the secondary classification of cancer-related IITs are shown in Figure 3b. Of these, lung cancer (15.5%, 1267/8199) was the most frequently studied cancer type, followed by breast cancer (9.5%, 783/8199), colorectal cancer (9.2%, 752/8199), and hepatic cancer (9.1%, 746/8199). In addition, 5 of the top 10 cancer types were digestive tumors, namely colorectal cancer, liver cancer, gastric cancer, esophageal cancer, and pancreatic cancer, accounting for 33.9% (2779/8199).
As shown in Figure 3c, the distribution of patients by age showed that, of the 303 IITs in which only adult patients (aged ≥18, <65 years) were included, patients with breast cancer (21.5%, 65/303), leukemia (14.5%, 44/303), cervical cancer (8.6%, 26/303), and nasopharyngeal cancer (8.6%, 26/303) were the most common; in 44 IITs in which only elderly patients (≥65 years) were included, digestive tumors were the most studied (52.3%, 23/44). In the primary classification, there were a total of 52 IITs on pediatric tumors, most of which were on hematological tumors, with IITs on leukemia accounting for 48.1% (25/52) and lymphoma accounting for 11.5% (6/52) of studies. With the exception of studies on pediatric tumors, 798 studies included patients aged 0–17 years, with the highest proportion of 59.4% (19/32) in bone tumors, followed by 51.2% (186/363) in leukemia, and 25.9% (91/352) in lymphoma.
We integrated the phase, randomization, masking, and enrollment of cancer-related IITs on the two registration websites (Supporting Information: Table S2). Phase 0 (namely pilot studies) was unique to ChiCTR, which mainly included diagnostic studies (n = 99), interventional studies (n = 333), and observational studies (n = 216). All 56 early phase 1 studies came from CT.gov, and were mainly focused on drugs (n = 27) and biological agents (n = 18). There were 3898 phase 1 to phase 4 studies, among which phase 2 accounted for the majority, at 39.0% (1521/3898). Most phase 3 studies adopted randomized methods (92.5%, 577/624), whereas the use of blinded settings was generally low (17.1%, 107/624). The proportion of randomized and double-blind design was only 8.0%, of which phase 3 was the highest at 16.0%. A notable gap between the average and median of recruited patients in phase 4 was attributed to the fact that one study recruited 200,000 patients. There were 437 clinical studies with sample sizes ≥1000, of which 29.1% (127/437) were interventional studies and 51.5% (225/437) were observational studies. Among the studies with large sample sizes, the top five cancer-related clinical studies were focused on colorectal cancer (n = 62), lung cancer (n = 60), breast cancer (n = 54), gastric cancer (n = 37), and hepatic cancer (n = 36). The differences in other classifications of study design between CT.gov and ChiCTR are shown in Supporting Information: Tables S3 and S4.
3.3 Drug classification of interventional cancer-related IITs
A total of 5215 interventional studies were classified according to specific interventional measures. Cancer-related IITs were mainly focused on drugs (44.2%, 2305/5215), procedures (14.9%, 775/5215), and combination treatment (21.0%, 1093/5215). Behavioral intervention and diet therapy, as supplement treatment, accounted for a relatively small proportion of studies: 0.9% (49/5215) and 1.1% (59/5215), respectively. In addition, 12 cancer-related IITs adopted gene therapy as an intervention.
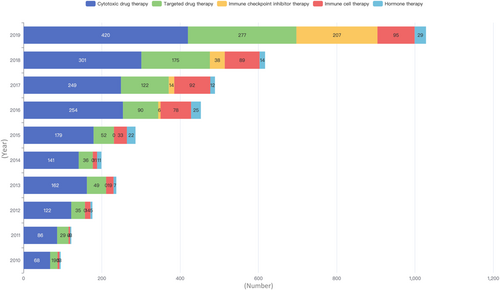
As shown in Figure 4 and Supporting Information: Table S5, the overall development of interventional studies on different drug types (including combination administration) over the past decade appeared to increase year on year, but this trend was not significant for hormone therapy. Cytotoxic drug therapy, as conventional treatment, was the focus of the largest number of studies, and cytotoxic drugs in combination with targeted drugs were the most common studies among combination therapeutic strategies. In 2016, studies on immune checkpoint inhibitors began to appear, with a significant year-on-year growth trend for combination studies with cytotoxic, targeted, and hormone agents. For immune cell therapy, a total of 440 studies have been carried out in the past decade.
3.4 Geographic distribution of studies
Although cancer-related IITs were carried out in 143 cities of 30 provinces/municipalities in mainland China, a geographic trend toward study location in economically developed cities (Shanghai, Beijing, and Guangdong) was apparent; these studies represented half of the total (51.4%, 4212/8199), while fewer than 10 studies took place in Qinghai, Ningxia, and Hainan, with smaller populations (Table 1). Cancer-related IITs in each province were mainly concentrated in the capital city (see Supporting Information: Videos S3 and S4). Studies carried out in 19 capital cities (excluding municipalities) accounted for more than 80% of all studies in their respective province, of which IITs in four provinces were carried out only in medical and scientific institutions in the provincial capital.
| Provinces/cities | Number | Provinces/cities | Number |
|---|---|---|---|
| Shanghai | 1472 | Guangxi | 134 |
| Beijing | 1412 | Anhui | 111 |
| Guangdong | 1328 | Hebei | 103 |
| Zhejiang | 449 | Heilongjiang | 88 |
| Sichuan | 438 | Jilin | 70 |
| Jiangsu | 402 | Guizhou | 43 |
| Chongqing | 284 | Xinjiang | 41 |
| Hubei | 277 | Gansu | 40 |
| Shandong | 273 | Jiangxi | 32 |
| Shaanxi | 219 | Shanxi | 26 |
| Tianjin | 211 | Yunnan | 16 |
| Henan | 204 | Inner Mongolia | 12 |
| Fujian | 198 | Qinghai | 7 |
| Liaoning | 151 | Ningxia | 7 |
| Hunan | 145 | Hainan | 6 |
- Abbreviations: ChiCTR, Chinese Clinical Trial Registry; CT.gov, ClinicalTrials.gov; IITs, investigator-initiated trials.
High regional incidence of specific tumor types had a strong correlation with the number of clinical studies performed in that region. Nasopharyngeal cancer occurs with a high incidence rate in Guangdong and Guangxi provinces, and studies on this cancer type accounted for 44.9% (133/296) and 9.1% (27/296) of the total nasopharyngeal cancer studies performed nationwide, ranking first and second, respectively. In Henan Province, which has a high incidence of esophageal cancer, 204 IITs were performed in the past decade, of which 57 were studies on esophageal cancer, accounting for 27.9% (57/204) and 12.6% (57/453) of the total clinical esophageal cancer studies in Henan Province and nationwide, respectively.
3.5 Funding type
Cancer-related IITs were divided into nine categories according to funding category (Figure 5). For 114 studies, more than one funding source was reported. Additionally, 14.3% (1176/8199) of IITs received government funding, with the average funding rate fluctuating by around 13% over 10 years.
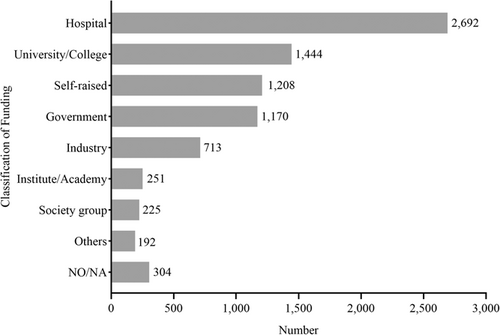
Although over 30% of funding for cancer-related IITs came from only four provinces, all these provinces carried out less than 50 studies. However, in Guangdong province, which was among the top three provinces for number of IITs performed, only 8.1% of cancer-related IITs received government fundings, while the majority of studies (35.7%; 474/1328) received funds from colleges and universities, twice the national average. Across all studies, the majority were funded by hospitals, accounting for 33.4% (2739/8199) in total; the hospital funding rate exceeding 40% in seven provinces. Additionally, 18.7% (1534/8199) of studies required funds raised by the investigators. Few studies (2.9%, 237/8199) were financed by social funds (such as nonprofit foundation), and 8.8% (722/8199) of studies were funded by industry.
3.6 Chronological shifts in IITs characteristics
The characteristics of IITs of the periods from 2010 to 2014 (n = 1630) and from 2015 to 2019 (n = 6569) are listed in Table 2. Compared with trials in 2010–2014, a higher proportion of trials in 2015–2019 were registered before the first participants enrolled (64.9% [4264 of 6569] vs. 40.2% [656 of 1630], p < 0.001) and had a data monitoring committee (DMC)(44.5% [2921 of 6569] vs. 40.0% [652 of 1630], p = 0.001).
| Characteristic | No./total no. (%) | p Value | |
|---|---|---|---|
| 2010–2014 | 2015–2019 | ||
| Registration before participant enrollment | 656/1630 (40.2) | 4264/6569 (64.9) | <0.001 |
| With a DMC | 652/1630 (40.0) | 2921/6569 (44.5) | 0.001 |
| Phase | 1036/1630 (63.6) | 2862/6569 (43.6) | <0.001 |
| 1 | 102/1036 (9.8) | 328/2862 (11.5) | 0.155 |
| 1|2 | 82/1036 (7.9) | 300/2862 (10.5) | 0.017 |
| 2 | 404/1036 (39.0) | 1117/2862 (39.0) | 0.985 |
| 2|3 | 53/1036 (5.1) | 106/2862 (3.7) | 0.049 |
| 3 | 199/1036 (19.2) | 425/2862 (14.8) | 0.001 |
| 4 | 196/1036 (18.9) | 586/2862 (20.5) | 0.284 |
| Allocation (randomized, phase 2–phase 4) | 534/852 (62.7) | 1151/2234 (51.5) | <0.001 |
| Blindinga | 185/885 (21.0) | 538/2727 (19.7) | 0.448 |
| Ageb | |||
| <18 | 180/1630 (28.6) | 668/6569 (10.2) | 0.300 |
| ≥65 | 1432/1630 (87.9) | 5432/6569 (82.7) | <0.001 |
| Interventions | 829/1184 (70.0) | 2873/4031 (71.3) | 0.403 |
| Cytotoxic drug therapy | 579/1184 (48.9) | 1403/4031 (34.8) | <0.001 |
| Targeted drug therapy | 168/1184 (14.2) | 716/4031 (17.8) | 0.004 |
| Immune checkpoint inhibitor therapy | 0/1184 (0.0) | 265/4031 (6.6) | <0.001 |
| Immune cell therapy | 53/1184 (4.5) | 387/4031 (9.6) | <0.001 |
| Hormone therapy | 29/1184 (2.4) | 102/4031 (2.5) | 0.876 |
| Funding (phase) | 917/1630 (56.3) | 2394/6569 (36.4) | <0.001 |
| 1 | 80/917 (8.7) | 291/2394 (12.2) | 0.005 |
| 1|2 | 67/917 (7.3) | 285/2394 (11.9) | <0.001 |
| 2 | 377/917 (41.1) | 969/2394 (40.5) | 0.739 |
| 2|3 | 53/917 (5.8) | 104/2394 (43.4) | 0.082 |
| 3 | 183/917 (20.0) | 382/2394 (16.0) | 0.006 |
| 4 | 157/917 (17.1) | 363/2394 (15.2) | 0.166 |
| Funding (cancer type) | 1417/1630 (86.9) | 5270/6569 (80.2) | <0.001 |
- Abbreviations: ChiCTR, Chinese Clinical Trial Registry; CT.gov, ClinicalTrials.gov; DMC, data monitoring committee; IITs, investigator-initiated trials.
- a Total no. was the number of randomized trials in blinding settings.
- b Studies that included patients <18 or ≥65 years of age were included in this analysis.
The proportion of randomized trials in 2015–2019 was lower than that in 2010–2014 (51.5% [1151 of 2234] vs. 62.7% [534 of 852], p < 0.001), whereas the proportion of blinded trials was comparable between the two periods. With regard to study phase, the proportion of trials in phase 3 decreased from 19.2% (199 of 1036) in 2010–2014 to 14.8% (425 of 2862) in 2015–2019 (p = 0.001), with no significant difference in other phases between the two periods. In terms of age selection, older adults were less likely to be enrolled in 2015–2019 (87.9% [1432 of 1630] vs. 82.7% [5342 of 6569], p < 0.001).
Differences in study intervention were also apparent. The proportion of trials involving cytotoxic drug therapy decreased from 48.9% (579 of 1184) in 2010–2014 to 34.8% (1403 of 4031) in 2015–2019 (p < 0.001), whereas an increase was observed in the proportion of trials involving targeted therapy (14.2% [168 of 1184] in 2010–2014 vs. 17.8% [716 of 4031] in 2015–2019, p = 0.004), immune checkpoint inhibitor therapy (0% [0 of 1184] vs. 6.6% [265 of 4031], p < 0.001), and immune cell therapy (4.5% [53 of 1184] vs. 9.6% [387 of 4031], p < 0.001).
With respect to study funding, the proportion of funded trials decreased from 56.3% (917 of 1630) in 2010–2014 to 36.4% (2394 of 6596) in 2015–2019 (p < 0.001), especially for phase 3 trials (20.0% [183 of 917] vs. 15.2% [382 of 2394], p = 0.006). However, the funding for phase 1 and phase 1/2 increased from 8.7% (80 of 917) in 2010–2014 to 12.2% (291 of 2394) in 2015–2019 (p = 0.005), and from 7.3% (67 of 917) to 11.9% (285 of 2394) (p < 0.001), respectively, while funding for other phases did not change substantially between the two periods.
4 DISCUSSION
Our study identified a total of 8,199 cancer-related IITs registered on CT.gov and ChiCTR and involved an in-depth analysis of multiple dimensions including tumor type, study design, intervention approach, geographic distribution, and funding, and to the best of our knowledge, is the first study to describe the overall landscape and development of IITs conducted in mainland China over the past decade.
As of 2005, the International Committee of Medical Journal Editors has required investigators to register their trials before participant enrollment as a precondition for publishing their work in member journals [12]. Currently, numerous medical journals require investigators to comply with this registration regulation, and delayed registration may hinder the successful publication of IITs. We are pleased to note that an increasing number of investigators in mainland China are complying with this regulation, with the proportion of trials registered before first participant enrollment increasing over time (from 40.2% in 2010–2014 to 64.9% in 2015–2019). In 2010, Robert et al. reported on the characteristics of clinical trials registered in the CT.gov database from 2007 to 2010, where oncology trials were also described as part of this 3-year “snapshot” [13]. In the present cross-sectional study, we characterized the scope and nature of oncology IITs registered in CT.gov and ChiCTR over a 10-year period. As with the previously published 2007–2010 snapshot, we examined trial design and funding source, with a new emphasis on analyzing trends over time. We also provided a detailed analysis of the classification of cancer types and an evaluation of the geographic distribution of studies in mainland China.
China is experiencing rapid transitions in health, including a heavy burden of cancer [14]. However, Chinese prevention and treatment guidelines for many diseases have been developed from evidence generated in western populations, or are based on expert opinion. Because of insufficient robust clinical research and data, China remains a country that imports rather than exports medical evidence [15]. To enhance clinical research, the Chinese government issued the “Three-year Action Plan for Cancer Prevention and Control in China (2015–2017)” in September 2015, which clearly stated that cancer-related clinical studies should be encouraged [16]. In the 13th Five-Year Plan (2016–2020) for the establishment of national and regional medical centers published in 2017, the National Health Commission emphasized that national medical centers should lead and conduct large, multicenter clinical trials focusing on major diseases in regions [17]. These national policies appear to have been effective. Taken together, the report published by the China Cancer Center in 2019 [7] and Figure 2 in our analysis show that the numbers of both industry-initiated and investigator-initiated cancer-related clinical trials have increased significantly over time, with an average annual increase of more than 30%, and the number of registered cancer-related IITs in 2019 was 11.4 times (2116/185) that in 2010. Furthermore, the proportion of cytotoxic drug therapy IITs, as a conventional treatment, decreased with the advent of immune/targeted therapies (down to 34.8% in 2015–2019 from 46.4% in 2010–2014), implying that recent key developments in oncological drug therapy, accompanied by progress in science and technology, might greatly improve cancer patient survival.
Regarding the classification of cancer types, IITs appear to closely follow the clinical treatment needs of most cancer patients. Malignant tumors of the digestive system, such as esophageal cancer, gastric cancer, and liver cancer, which are interlinked with unhealthy lifestyles in mainland China, are highly prevalent and were also the focus of most registered cancer-related IITs in the past decade. This observation is complementary to new drug research and development to enhance digestive tract cancers with life-threatening adverse outcomes unique to Chinese populations, as described by Li et al. [7]. The most recent epidemiological data show that, among the top five malignant tumors for incidence and death in China, digestive tract malignancies accounted for 3 of 5 and 4 of 5, respectively, in 2015 [18]. Cancer-related IITs also address rare cancers, and 3% of the IITs included in our analysis investigated rare cancers (“Others” in the secondary classification). Studies on rare forms of cancer are typically of lower commercial interest, but their initiation, whether as an observational study or an interventional study, are beneficial to patients. In addition, only 52 IITs focused on pediatric tumors, the incidence of which has been reported to be increasing at an annual rate of 2.8% in China, with a substantial burden of disease when compared with adult cancers and general pediatric diseases [19, 20]. In contrast, there remains a widening gap between the incidence of pediatric tumors and the number of clinical studies carried out in this area, and the initiation of IITs in children under the age of 18 years requires continual focus.
We also found that the development of clinical studies is closely related to local medical resources. With the exception of the Tibet Autonomous Region, cancer-related IITs have been carried out in 23 provinces, four autonomous regions, and four municipalities in mainland China. Cancer-related IITs were mainly concentrated in economically developed provinces and cities, and the eastern provinces carried out significantly more studies than the western provinces. The IITs carried out in each province were concentrated in its capital city, with most studies centered in a few high-profile hospitals. The tendency for cancer-related IITs to be centralized presents a certain challenge for recruiting patients, which could be addressed by increasing the number of study sites as with the clinical studies of new drugs initiated by industry. However, at present, few investigators invite external parties (such as contract research organizations) to manage their cancer-related IITs because of insufficient funding. Compared with other types of studies, cancer-related clinical studies tend to be more complicated to conduct. How to ensure the quality of the study in the sub-center and guarantee the safety of subjects requires close consideration by the study initiator.
It is well established that the first step to achieve reliable results in clinical studies lies in the design of the study. Serving as one of the largest registries of clinical trials and a useful tool to understand the clinical trial landscape, several analyses of registrations in CT.gov have been published, including in the fields of oncology [21-23], psychiatry [24], ophthalmology [25], infectious disease [26], and COVID-19 [27]. In the present study, we integrated the phase, enrollment, randomization, and masking of cancer-related IITs from the two registration websites and identified 3898 studies in phase 1 to phase 4, with phase 2 accounting for the majority (39.0%, 1521/3898), similar to the results of an analysis of Japanese IITs [11]. It is unfortunate that the proportion of randomized IITs has decreased in recent years and that the number of blinded studies was generally low, representing only 107 (17.2%) trials. The proportion of randomized and double-blind trials was only 8.0%, consistent with a systematic analysis in 2013 [23]. These findings might provide important insights into the quality of clinical trials. Despite increasing research in oncology, notable differences were found in other disease fields. High proportions of ophthalmology trials reported rigorous trial standards such as randomization (64.8%) and blinding (78.5%) [25]. A comprehensive analysis reported in the British Medical Journal investigated the dissemination of clinical trials and found that those reporting the use of randomization were more likely than non-randomized studies to be published in a journal article, and thus adopted as clinical evidence [28]. Furthermore, only 437 clinical studies enrolled 1000 or more subjects, of which only 29.1% were interventional studies. Although small sample sizes could be partially explained by shifts towards personalized, precision medicine, as many genetic and pathophysiology studies target specific sub-populations [29, 30], as well as by selection pressure [23], it should be noted that trials with small sample sizes are unlikely to be informative in many other settings, such as establishing the effectiveness of treatments with modest effects and comparing effective treatments to enable better decision-making in practice [31, 32]. Additionally, establishment of a DMC is important to assure the safety of enrolled patients, maintain the equipoise of the trial, and ensure scientific rigor and trial integrity [33]. Although the increase in the proportion of trials with a DMC from 2015 to 2019 (44.5%) compared with 2010–2014 (40.0%) is encouraging, the proportion is nevertheless modest at <50.0%. Hence, the appropriate design of high-quality cancer-related studies to achieve reliable results in a multi-center setting is a complex undertaking for prospective investigators.
Despite the key findings identified in our analysis, our study had several limitations. First, our analysis was based on data obtained from two clinical trial registration websites. The accuracy and timeliness of information entry may have affected the results. In addition, the number of observational IIT studies may have been underestimated, as the registration of observational studies is not mandatory. Second, inconsistencies in study design and the classification of intervention measures between these two websites prevented the data from being combined for analysis, which impacted the presentation and analysis of the results. We propose that the WHO International Clinical Trials Registry Platform and CT.gov may be better aligned in this regard, including the definition and classification of interventional and observational studies. Finally, it would be relevant to investigate whether the final results of registered clinical studies were published, and to examine consistencies between studies; however, such analyses were not in scope for the present study.
5 CONCLUSIONS
Our analysis of IITs registered on CT.gov and ChiCTR described the overall landscape and development of cancer-related IITs conducted in mainland China. The past decade has seen significant progress in study quantity, although limitations on quality assurance, regional imbalances, and funding allocation remain. Our findings should be considered in the development of benchmarks and for facilitating improvements in this important area of research.
AUTHOR CONTRIBUTIONS
Ye Cao: Conceptualization (lead); project administration (lead); resources (lead); supervision (lead); validation (equal); writing—review and editing (lead). Lin-Miao Ye: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); validation (equal); writing—original draft (equal); writing—review and editing (equal). Zhong Fan: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); validation (equal); writing—original draft (equal); writing—review and editing (equal). Wei Yang: Data curation (supporting); investigation (supporting); resources (supporting). Li-Ying Chen: Investigation (supporting); methodology (supporting). Yun Mei: Visualization (lead). De-Ying He: Resources (equal). Wen-Jin Mo: Resources (equal).
ACKNOWLEDGMENTS
We thank Yanhua Xu from Jiangsu Hengrui Pharmaceuticals Co., Ltd., Shanghai, China for her careful guidance during our writing of this paper.
CONFLICT OF INTEREST STATEMENT
Y.M. is employed by Yidu Tech Inc., Beijing, China. W.M. and D.H. are employed by Guangzhou Yushi Medicinal Technology Co., Ltd., Guangzhou, China. The remaining authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All data analyzed in the study are available from a public registration database. Study-related datasets can be obtained upon request by contacting the corresponding author.




