TRIP13: A promising cancer immunotherapy target
Shengnan Jing and Liya Zhao contributed equally to this work and shared the co-first authorship.
Abstract
The tumor microenvironment (TME) facilitates tumor development through intricate intercellular signaling, thereby supporting tumor growth and suppressing the immune response. Thyroid hormone receptor interactor 13 (TRIP13), an AAA+ ATPase, modulates the conformation of client macromolecules, consequently influencing cellular signaling pathways. TRIP13 has been implicated in processes such as proliferation, invasion, migration, and metastasis during tumor progression. Recent studies have revealed that TRIP13 also plays a role in immune response suppression within the TME. Thus, inhibiting these functions of TRIP13 could potentially enhance immune responses and improve the efficacy of immune checkpoint inhibition. This review summarizes the recent research progress of TRIP13 and discusses the potential of targeting TRIP13 to improve immune-based therapies for patients with cancer.
Abbreviations
-
- BRCA
-
- breast cancer
-
- HNSC
-
- head and neck squamous cell carcinoma
-
- KIRC
-
- kidney renal clear cell carcinoma
-
- LUAD
-
- lung adenocarcinoma
-
- STAD
-
- stomach adenocarcinoma
-
- THCA
-
- thyroid carcinoma
-
- THYM
-
- thymoma
1 INTRODUCTION
Thyroid hormone receptor interactor 13 (TRIP13) is a member of the AAA+ ATPase family that generates mechanical forces through ATP hydrolysis reactions. Existing as homohexamers, TRIP13 uses ATP as its energy source and interacts with its analog pontocerebellar hypoplasia type 2 (PCH2). PCH2 binding to hsp70-Hsp90 organizing protein 1 (Hop1) induces structural changes, displacing Hop1 from DNA [1]. TRIP13/PCH2 functions as an ATP-dependent hydrolase and uses phosphate hydrolysis to generate energy for conformational changes, exerting mechanical force on its substrate Hop1 [2]. TRIP13 was also identified as a kinetochore protein and it interacts with the silencing protein MAD2L1 binding protein (also known as p31comet) [3]. In cancer cells, TRIP13 was reported to interact with mitotic arrest deficient 2 (MAD2), actinin alpha 4 [4], NIMA-related kinase 2 [5], epidermal growth factor receptor [6], and fibroblast growth factor receptor 4 (FGFR4) [7], thereby promoting tumor progression. Its role in ensuring the accurate alignment of chromosomes during mitosis aligns with its association with various cancers. TRIP13 is upregulated in several types of cancers, including colorectal cancer [8], head and neck cancer [9], breast cancer [10], lung cancer [11], liver cancer [12], prostate cancer [13], multiple myeloma [14], and human chronic lymphoblastic leukemia [15]. Overexpression of TRIP13 has been linked to accelerated tumor growth in squamous cell cancer of the head and neck [9] and lung adenocarcinoma [16]. Blocking TRIP13 has been demonstrated to slow tumor growth in hepatocellular carcinoma, head and neck cancer, and colon cancer [17, 18]. Moreover, in cutaneous melanoma [19], prostate cancer [13], and lung adenocarcinoma [16], TRIP13 plays a crucial role in maintaining tumor “stem” or triggering cells and facilitating their movement. These findings underscore the significance of TRIP13 in tumor progression.
TRIP13 also has a significant role in the immune system. A TRIP13-like ATPase was identified as instrumental in regulating bacterial defense against bacteriophages [20]. Previous studies also underscore TRIP13's substantial involvement in the immune system's response to tumors. This is achieved through TRIP13 regulation of the migration of normal cells to the tumor microenvironment (TME), thereby facilitating tumor survival. Among these cells are myelin stromal cells and immune cells including CD3+ T cells, CD4+ T cells, and CD8+ T cells, all of which tumors can exploit to modulate immune responses. While TRIP13's contribution to tumor growth has been extensively discussed [21], this review emphasizes recent findings on the emerging roles for TRIP13 in suppressing the immune response within the TME.
2 THE ROLE OF TRIP13 IN PHYSIOLOGICAL PROCESSES
TRIP13/PCH2 plays a significant role in mitosis, specifically during the transition from metaphase to anaphase and in the spindle assembly checkpoint (SAC). Its activity also influences the anaphase-promoting complex (APC) [22]. Before progressing into anaphase, the cell must ensure proper chromosome organization and biorientation to facilitate the smooth separation of sister chromatids. This intricate process requires multiple proteins for precise timing and reliable reactions.
Activating the APC is crucial for movement of sister chromatids but comes with a compromise in security during the metaphase-anaphase transition. Cell division cycle 20 (CDC20), which is usually inhibited by the mitotic checkpoint complex (MCC), triggers the APC. Mad2 is a mitotic checkpoint gene that encodes a protein that exists in two distinct isoforms: the open (O-) form and the closed (C-) form [22]. O-Mad2 undergoes a transformation to C-Mad2 upon kinetochore detachment. Subsequently, C-Mad2 binds to CDC20, inhibiting its function and impeding mitotic progression [23]. To proceed, disassembling the MCC is necessary, and this process is mediated by p31comet [24]. Structural mimicry is believed to occur, partly from the structural similarities between p31comet and C-Mad2 [25]. However, ATP is essential for this process, and TRIP13/PCH2 plays a role in this step (Figure 1). Thus, TRIP13/PCH2 plays a crucial function in activating the SAC and in the development of the MCC.
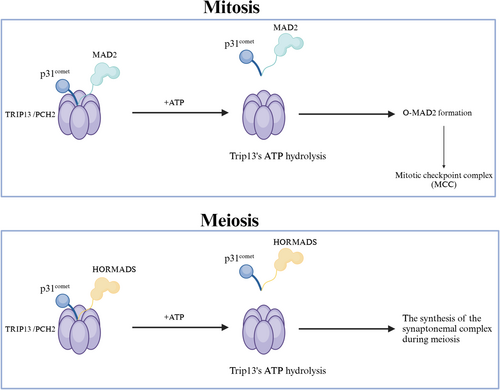
TRIP13/PCH2 is also implicated in the G2/prophase stage of meiosis [26] (Figure 1). TRIP13 influences the frequency of double-strand breaks, which are crucial in meiosis. Homologous recombination then requires the formation of a protein complex to guide proper chromosomal pairing. The meiotic checkpoint in budding yeast prevents chromosomal segregation in cases of recombination or chromosome synapsis deficiencies, as evidenced by induced mutations in the PCH2 gene [26].
TRIP13/PCH2 is vital for the synaptonemal complex (SC) formation, which is responsible for chromosome pair formation. Meiocytes lacking TRIP13 showed an increase in pericentric synaptic forks, a decrease in crossovers, and disrupted chiasma distribution (sites where homologous chromosomes contact) [27]. Synthesis of the SC during meiosis necessitates the removal of HORMA-domain proteins (HORMADS). PCH2 is essential for the SC formation by facilitating Hop1 removal from chromosomes [28]. TRIP13 facilitates the removal of various HORMADS, such as HORMAD1 and HORMAD2, in mouse spermatocytes [29]. These findings indicate the crucial and active role of TRIP13/PCH2 in eliminating multiple proteins during SC formation and subsequent meiosis.
3 THE ROLE OF TRIP13 IN CANCER
The role of TRIP13/PCH2 in maintaining correct chromosomal biorientation during mitosis should not be surprising given that it is linked to many cancers. TRIP13 overexpression promotes invasion and proliferation of squamous cell carcinoma of the head and neck [9]. Additionally, TRIP13 overexpression mitigates the mitotic delay induced by Mad2 overexpression, while TRIP13 downregulation exacerbated the effects of Mad2 overexpression. Both Mad2 and TRIP13 overexpression promote proliferation in cells and tumor xenografts, indicating the potential of TRIP13 inhibition as a therapeutic strategy for cancer treatment [24]. Additionally, Mad2 has been implicated in ovarian cancer treatment resistance [30]. Epithelial ovarian cancer (EOC) cell lines (SKOV-3, HEY, and OVCAR-3) exhibited higher TRIP13 expression compared with normal cell lines [31]. Knockdown of TRIP13 in EOC cells induced cell death, reduced cell invasion and migration, and hindered proliferation [31]. These findings indicates that TRIP13 aids tumor cell growth and highlights TRIP13 as a potential target for cancer treatment.
4 TRIP13 AND IMMUNE CELL INFILTRATION
4.1 Roles of infiltrating immune cells in the TME
Tumor-infiltrating immune cells (TIICs) are pivotal components of the TME and hold significant implications for both prognostic assessment and therapeutic interventions in patients with cancer [32-34]. Tumor-infiltrating lymphocytes (TILs) have been extensively studied as a specific subset of TIICs, and their association with positive outcomes in breast cancer has been previously documented [35-37]. Elevated levels of TILs within tumor masses correlated with improved overall survival and may serve as indicators of heightened responsiveness to chemotherapy or radiotherapy [38-44]. Furthermore, the presence of TILs has been associated with a reduced rate of distant recurrence and enhanced metastasis-free survival in various types of cancers [39, 45-47]. However, the relationship between TIL abundance and treatment response is not always consistent, indicating that the distinct immune phenotypes exhibited by TILs play a critical role in prognostic evaluation [35].
de Visser et al. [48] reported a potential association between B lymphocytes and tumor promotion. Their findings demonstrated that genetically removing mature B and T lymphocytes in a mouse model restricted the advancement of cancerous growths, as these tumors failed to attract innate immune cells. Moreover, the transfer of B lymphocytes or serum from HPV16 mice to B- and T-cell-deficient/HPV16 mice led to the restoration of innate immune cell infiltration into premalignant tissue, thereby reinstating conditions conducive to full malignancy. Furthermore, the substantial presence of regulatory T cells (Tregs) within various tumor types contributes to the establishment of an immunosuppressive microenvironment, thereby impeding an effective immune response against the tumor and promoting its growth [49-51]. The positive impact of inhibiting Treg cell functions has been observed in animal models, leading to enhanced T-cell-mediated antitumor responses and the regression of experimental tumors [52, 53].
Like TILs, tumor-associated macrophages (TAMs) represent a distinct immune population crucial in regulating the interaction between cancer and the immune system. Clinicopathological studies have indicated the adverse clinical implications associated with TAM accumulation [54-64]. Additionally, natural killer (NK) cells exert a beneficial influence on immunosurveillance [65-67]. Early investigations in animal models revealed the pivotal role of NK cells in impeding the dissemination of cancer cells to distant sites [68-72]. Studies demonstrated that the clearance of tumor cells in the lungs primarily relied on NK cells. Additionally, the rate of NK cell-mediated elimination of tumor cells in the lungs was inversely related to the formation of experimental metastases in the lungs and other organs after intravenous injection of tumor cell suspensions [68-71]. Elevated levels of tumor-infiltrating NK cells have been linked to improved prognosis in certain types of solid tumors. However, the immunosuppressive TME hampers the efficacy of these cells, thereby facilitating cancer progression [72]. In the TME, dendritic cells (DCs) are highly effective antigen-presenting cells that initiate primary immune responses against carcinoma [73]. Mast cells, myeloid-derived suppressor cells, and monocytes have been associated with an inflammatory microenvironment that promotes tumor growth [57, 74, 75]. Moreover, elevated neutrophil counts correlated with poor prognoses across various carcinoma types [59]. Eosinophils play a multifaceted function within the TME. Leveraging a diverse array of mediators and receptors, eosinophils actively engage in both innate and adaptive immune processes, including type 1 and type 2 immunity, thereby exerting influence over the TME and subsequent tumor outcomes [76]. Activated eosinophils in the TME modulate tumor growth by orchestrating the behavior of other immune cells through the release of cytokines [76].
Taken together, these results demonstrate that the presence of infiltrating immune cells within the TME holds significant prognostic implications for the survival outcomes of patients with cancer.
4.2 TRIP13 inhibits antitumor immune responses
Previous studies have indicated that TRIP13 may have a notable effect on tumor progression by activating immune cells that infiltrate the tumor. Among these cells, tumor-infiltrating CD8+ T cells are recognized as the key players in exerting antitumor effects. Granzyme B and perforin are essential cytotoxic components in NK cells and cytotoxic T lymphocytes, facilitating tumor cell apoptosis [77]. Inhibition of TRIP13 leads to upregulation of cytotoxic mediators, thereby activating an antitumor immune response. Tumor cells with TRIP13 knockdown exhibit increased granzyme B activation [7]. These findings suggest that the cytotoxic effects of TRIP13 on tumor cells may be attributed to the increased secretion of these mediators. Furthermore, a previous study of a myeloma model demonstrated that the suppression of TRIP13 leads to a notable increase in the infiltration of CD3+, CD4+, and CD8+ T cells [78]. In hepatocellular carcinoma, elevated TRIP13 expression was associated with the enhanced infiltration of Th2 cells and reduced infiltration of neutrophils, Th17 cells, and dendritic cells [79].
DCZ0415, a small molecule inhibitor of TRIP13, has been shown to significantly enhance the secretion of cytotoxic mediators, including granzyme B, perforin, and interferon-γ. This increased secretion may be responsible for the observed cytotoxic effects of DCZ0415 against murine MC38 cells. Furthermore, the combination of DCZ0415 with immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA4) in tumors increased cytotoxicity and promoted tumor regression [7]. In an immunocompetent syngeneic pancreatic ductal adenocarcinoma model, treatment with DCZ0415 resulted in an enhanced immune response. This was achieved through the downregulation of PD-1 or programmed death-ligand 1 (PD-L1) expression, upregulation of granzyme B/perforin expression, and augmentation of CD3/CD4 T cell infiltration [80]. Moreover, DCZ0415 augmented the antitumorigenic and antimetastatic properties of gemcitabine by inhibiting proliferation and angiogenesis, stimulating apoptosis, and boosting the immune response [80]. These findings indicate that TRIP13 depletion significantly impedes tumor progression by fostering increased immune cell infiltration.
4.3 The molecular mechanism of TRIP13 in antitumor immune responses
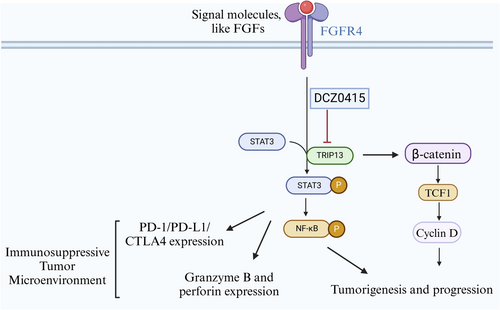
TRIP13 inhibition presents a promising avenue for immunotherapy against tumors, although further elucidation of its mechanism in cancer immunotherapy is warranted. Administration of DCZ0415 to immunocompetent myeloma models resulted in an upregulation of immune cell populations (CD3, CD4, and CD8) and an inactivation of nuclear factor-κB and Wnt/β-catenin signaling, suggesting the immunotherapeutic potential of TRIP13 inhibition [78]. TRIP13 blockade led to the signal transducer and activator of transcription 3 (STAT3) inactivation, promoting antitumor immunity, and impeding tumor growth [7]. Previous studies have shown the pivotal role of STAT3 in the regulation of the antitumor immune response through various mechanisms, including suppression of the production of immunosuppressive factors (Tregs), upregulation of immune checkpoint molecules PD-1 and CTLA-4, and inhibition of the antitumorigenic functions of CD8+ T cells [81]. Additionally, tumor progression has been linked to heightened activation of the FGFR4-STAT3 axis, which is induced by elevated TRIP13 levels and can be inhibited by DCZ0415 [7]. Subsequent studies showed that DCZ04145 inhibits the TRIP13-FGFR4-STAT3 axis in colorectal cancer, thereby inhibiting cancer progression and metastasis and stimulating the antitumor immune response [7]. These findings support the potential of targeting the TRIP13-FGFR4-STAT3 axis as a promising strategy for tumor immunotherapy (Figure 2).
5 TRIP13 IS A POTENTIAL REGULATOR OF TUMOR IMMUNE INFILTRATION
In addition to the prior studies highlighting TRIP13's role in regulating infiltrating T cells, we conducted further investigations into its putative immune regulatory functions using database analyses. Using the TIMER 2.0 database, we explored the association between TRIP13 expression and immune infiltration levels across various types of cancer. A significant negative correlation was observed between TRIP13 expression and CD8+ T cells in six out of 40 distinct cancer types (adjusted p < 0.05) (Supporting Information S1: Table S1). Notably, a positive correlation was detected between TRIP13 expression and CD8+ T cells in lung adenocarcinoma (LUAD) (adjusted p < 0.01). Furthermore, TRIP13 expression exhibited a significant positive correlation with CD4+ T cells in 10 out of 40 cancer types (adjusted p < 0.05).
TRIP13 expression exhibited a significant positive correlation with Tregs in kidney renal clear cell carcinoma (KIRC), kidney chromophobe, thyroid carcinoma (THCA), and liver hepatocellular carcinoma. Conversely, a significant negative correlation was observed between TRIP13 expression and the presence of Tregs and B cells across 12 different cancer types (adjusted p < 0.05). TRIP13 expression showed a significant positive correlation with B cells in THCA (adjusted p < 0.001) and with neutrophils in stomach adenocarcinoma (STAD), KIRC, and kidney renal papillary cell carcinoma (adjusted p < 0.05).
Notably, TRIP13 expression exhibited a significant negative correlation with monocytes in 8 different cancer types (adjusted p < 0.05), while showing a significant positive correlation with macrophage M1 cells in 13 types of cancers (adjusted p < 0.05) and macrophage M2 cells in prostate adenocarcinoma (adjusted p < 0.001). However, it exhibited a negative correlation with macrophage M1 cells in thymoma (THYM) (adjusted p < 0.05) and macrophage M2 cells in six types of cancers (adjusted p < 0.05). Furthermore, TRIP13 expression exhibited a significant positive correlation with DCs in THCA and rectum adenocarcinoma (adjusted p < 0.05), while showing a significant negative correlation with DCs in luminal A breast cancer (BRCA-LumA), LUAD, KIRC, and mesothelioma (adjusted p < 0.05). TRIP13 expression showed a significant positive correlation with NK cells in colon adenocarcinoma and testicular germ cell tumors (adjusted p < 0.05) and a significant negative correlation with NK cells in human papillomavirus-negative head and neck squamous cell carcinoma (HNSC-HPV−), BRCA-LumA, HNSC, STAD, and THYM (adjusted p < 0.05). TRIP13 expression displayed a significant negative correlation with mast cells in 11 different cancer types (adjusted p < 0.05). No significant correlation was observed between TRIP13 expression and eosinophil cells. Collectively, these findings strongly suggest that TRIP13 may serve as a significant regulator of tumor immune infiltration in the context of cancer therapy.
6 DISCUSSION AND CONCLUSIONS
As a protein involved in regulating chromosomal alignment during mitosis, the association of TRIP13/PCH2 with a variety of malignancies is not surprising. Studies have indicated that TRIP13 may represent an attractive target for cancer therapy. Additionally, TRIP13 plays a crucial function within the immune system. The TME consists of a diverse array of innate and adaptive immune cells, cancer cells, endothelial cells, and fibroblasts, including B and T cells, dendritic cells, myeloid-derived suppressor cells, mast cells, NK cells, neutrophils, eosinophils, and macrophages (M1/M2) [82, 83]. Cancer development and progression are significantly influenced by the TME. Some evidence suggests that cellular interactions between immune cells and cancer cells may promote cancer cell proliferation. Previous studies have also identified the crucial functions of TRIP13 in tumor immunity, specifically in facilitating tumor survival by modulating the recruitment of immune cells, including CD3+ T cells, CD4+ T cells, and CD8+ T cells, to the TME. As demonstrated in earlier studies, the depletion of TRIP13 made it easier for immune responses to be activated within the TME, ultimately impeding tumor progression [78]. TRIP13 in tumor cells possesses the ability to suppress immune responses, thereby promoting tumor progression.
More research is required to determine whether TRIP13-mediated tumor growth leads to the upregulation of immune checkpoint inhibitors beyond PD-1/PD-L1/CTLA-4. Identifying the specific genes within immune cells that are regulated by TRIP13 is also critical. The exploration of these genes holds promise for the development of immunotherapeutic targets aimed at cancer prevention. Furthermore, to advance TRIP13-based cancer immunotherapy, it is imperative to gain a deeper understanding of TRIP13's role in other infiltrating immune cells, such as regulatory T cells and macrophages. Fully elucidating the involvement of TRIP13 in the function and regulation of these immune cells will be essential for developing effective immunotherapeutic strategies targeting TRIP13 for cancer treatment.
TRIP13 facilitated the upregulation of STAT3 expression, hence inducing anticancer immunity and impeding tumor advancement [7]. Tumor progression was observed as a result of elevated TRIP13 levels, which led to hyperactivation of the FGFR4-STAT3 axis [7]. DCZ0415 inhibits this axis, thereby inhibiting the progression and metastasis of colorectal cancer while stimulating the antitumor immune response [7]. These findings indicate that targeting the TRIP13-FGFR4-STAT3 axis holds promise as a strategy for tumor immunotherapy. By directing the development of targeted pharmacologic strategies to reduce or eliminate the effects of infiltrating immune suppression in patients with cancer, a targeted TRIP13-FGFR4-STAT3 therapy enhances the efficacy of therapeutic cancer vaccines and other immune cell-based cancer immunotherapies. A targeted TRIP13-FGFR4-STAT3 therapy may enhance the efficacy of therapeutic cancer vaccines and other immune cell-based cancer immunotherapies and reduce or eliminate the effects of infiltrating immune suppression in cancer patients.
A recent study showed that blocking TRIP13 with DCZ0415 inhibits PD-1/PD-L1 expression and increases T cell infiltration into the TME via modulation of the FGFR4/STAT3 axis, hence fostering antitumor immunity and preventing tumor growth [80]. Therefore, the combination of DCZ0415 with first-line cancer therapy medicines holds significant promise as a therapeutic approach for treating cancer. The combined therapy not only decreased tumor growth but also increased the infiltration of cytotoxic T cells while it decreased PD-1 expression, therefore enhancing antitumor immunity. One limitation of DCZ0415 is its effectiveness at high concentrations/doses [7, 80]. Hence, developing novel, safe, and potent derivatives of DCZ0415 or exploring alternatives such as anti-TRIP13 antibodies or TRIP13 short hairpin RNA viruses is critical. These innovations could be instrumental in future clinical studies, offering improved efficacy and broader applicability in cancer therapy.
AUTHOR CONTRIBUTIONS
Shengnan Jing: Project administration (equal); writing—original draft (equal). Liya Zhao: Investigation (equal). Liwen Zhao: Software (equal). Yong-Jing Gao: Supervision (equal); writing—review and editing (equal). Tianzhen He: Investigation (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data from this study are available upon request from the authors.




