Cardiovascular toxicity with CTLA-4 inhibitors in cancer patients: A meta-analysis
Abstract
Background
With the emergence of cytotoxic T lymphocyte-associated protein-4 (CTLA-4) inhibitors, the outcomes of patients with malignant tumors have improved significantly. However, the incidence of cardiovascular adverse events has also increased, which can affect tumor treatment. In this study, we evaluated the incidence and severity of adverse cardiovascular events caused by CTLA-4 inhibitors by analyzing reported trials that involved CTLA-4 inhibitor therapy.
Methods
Randomized clinical trials published in English from January 1, 2013, to November 30, 2022, were searched using the Cochrane Library and PubMed databases. All included trials examined all grade and grades 3–5 cardiac and vascular adverse events. These involved comparisons of CTLA-4 inhibitors to placebo, CTLA-4 inhibitors plus chemotherapy to chemotherapy alone, CTLA-4 inhibitors combined with PD-1/PD-L1 inhibitors to PD-1/PD-L1 inhibitors alone, and CTLA-4 inhibitors plus target agent to PD-1/PD-L1 inhibitors plus target agent. The odds ratio (OR) and corresponding 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel method.
Results
Overall, 20 trials were included. CTLA-4 inhibitors significantly increased the incidence of all-grade cardiovascular toxicity (OR = 1.33, 95% CI: 1.00–1.75, p = 0.05). The incidence of all-grade cardiovascular toxicity increased in malignant tumor patients who received single-agent CTLA-4 inhibitors (OR = 1.73, 95% CI: 1.13–2.65, p = 0.01), as well as the incidence rate of grades 3–5 cardiovascular adverse events (OR = 2.00, 95% CI: 1.08–3.70, p = 0.03). Compared with the non-CTLA-4 inhibitor group, CTLA-4 inhibitors plus chemotherapy, PD-1/PD-L1 inhibitors, or target agent did not significantly affect the incidence of cardiac and vascular toxicity. The incidence of grades 3–5 cardiac failure, hypertension, pericardial effusion, myocarditis, and atrial fibrillation were much higher among patients exposed to CTLA-4 inhibitor, but the data were not statistically significant.
Conclusion
Our findings suggest that the incidence rate of all cardiovascular toxicity and severe cardiovascular toxicity increased in patients who were administered CTLA-4 inhibitors. In addition, the risk of serious cardiovascular toxic events was independent of the type of adverse event. From these results, physicians should assess the benefits and risks of CTLA-4 inhibitors when treating malignancies.
Abbreviations
-
- BTC
-
- biliary tract cancer
-
- CHF
-
- congestive heart failure
-
- CIs
-
- confidence intervals
-
- CTCAE
-
- common toxicity criteria
-
- CTLA-4
-
- cytotoxic T lymphocyte-associated protein-4
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- ES-SCLC
-
- extensive-stage small-cell lung cancer
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- HTN
-
- hypertension
-
- ICIs
-
- immune checkpoint inhibitors
-
- MeSH
-
- Medical Subject Headings
-
- MI
-
- myocardial infarction
-
- MSI
-
- microsatellite instability
-
- NSCLC
-
- non-small-cell lung cancer
-
- OR
-
- odds ratio
-
- PD-1/PD-L1
-
- programmed cell death protein 1/programmed cell death ligand-1
-
- RCC
-
- renal cell carcinoma
1 INTRODUCTION
The discovery of tumor-associated antigens and their recognition by activated T cells [1] has revolutionized the treatment methods for many malignant tumors and significantly improved patient outcomes [2]. Cytotoxic T lymphocyte-associated protein-4 (CTLA-4) is expressed in activated T cells and can inhibit the uncontrolled proliferation of T cells after binding to B7 [3, 4]. A CTLA-4 inhibitor, one type of immune checkpoint inhibitor (ICI), can bind to CTLA-4 and restore the T cell-mediated immune response against tumor cells. Two specific CTLA-4 inhibitors, ipilimumab, and tremelimumab, have been shown to be effective in patients with metastatic melanoma, nonsmall-cell lung cancer (NSCLC), relapsed malignant pleural mesothelioma, advanced pancreatic cancer [5-8], hepatocellular carcinoma [9], and malignant mesothelioma [10] when used as a monotherapy or in combination with other drugs. However, with the wide application of CTLA-4 inhibitors, increasing studies have suggested that they can cause a variety of immune-related toxicities by enhancing the systemic immune response. Cardiovascular toxicity is one of the most significant and severe complications [3]. Cardiovascular toxicities of CTLA-4 inhibitors include congestive heart failure, pericardial effusion, myocardial infarction, myocarditis, and hypertension [11]. As a result of these toxicities, cancer treatment may be delayed or stopped, and the subsequent outcomes may be worse. Therefore, it is essential to assess the incidence and severity of cardiovascular toxicity when using CTLA-4 inhibitors. In this meta-analysis, we evaluated the incidence of various cardiovascular toxicities associated with using CTLA-4 inhibitors alone or combined with other targeted agents to guide their administration to cancer patients better.
2 PATIENTS AND METHODS
2.1 Search methods and study selection
We searched all human randomized control trials published in English between January 1, 2013, and November 30, 2022, in the Cochrane Library and PubMed databases. The Medical Subject Headings (MeSH) terms and their entry terms were “ipilimumab,” “tremelimumab,” “CTLA-4 inhibitors,” and “CTLA-4.” Only studies with the sole difference between the experimental and control groups being the use of CTLA-4 inhibitors were included.
These studies included trials that compared single-agent CTLA-4 inhibitors to placebo, CTLA-4 inhibitors combined with chemotherapy to chemotherapy alone, CTLA-4 inhibitors combined with another ICI (PD-1/PD-L1 inhibitors) to PD-1/PD-L1 inhibitors alone, and CTLA-4 inhibitors combined with target agent to PD-1/PD-L1 inhibitors combined with target agent. Only trials that mentioned cardiovascular side effects were included. The abstracts and duplicate trials were deleted. The titles and abstracts of identified publications were carefully screened by two researchers independently. Full-text evaluations were conducted for any publications that the researchers thought were potentially relevant.
2.2 Data collection
Data were collected on all grades and grades 3–5 cardiovascular toxicities, which included myocardial infarction, cardiac failure, pericardial effusion, cardiac arrest, myocarditis, hypertension, and atrial fibrillation. The type and dose of ICI, tumor type, and number of study patients were noted. Common Toxicity Criteria (CTCAE) version 5.0 was used to classify the cardiovascular adverse events. A grade 1 or 2 cardiac disorder means that the patient is asymptomatic or has mild symptoms and does not require urgent intervention, respectively. A grade 3 adverse event means that the patient has severe symptoms or onset of symptoms that require urgent intervention. Grade 4 adverse events are usually life-threatening, while a grade 5 adverse event indicates death.
2.3 Statistical analysis
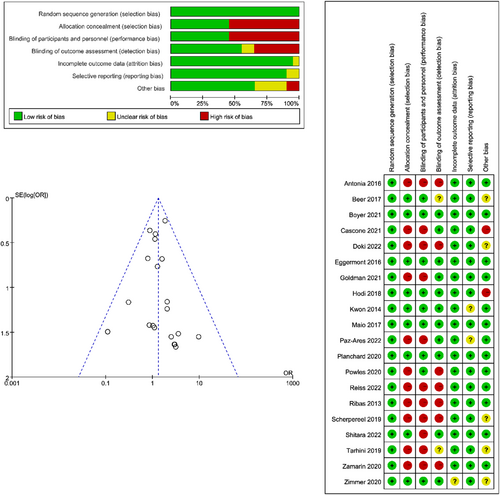
We assessed the quality of each included study and performed our analysis using RevMan v.5.4. The article quality was evaluated by detecting the following biases: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The bias risk was also summarized. The method of effect estimation was determined according to I2. When I2 < 50%, we used the fixed-effect model. Otherwise, a random-effect model was used. A p ≤ 0.05 (double-tailed) was considered statistically significant.
3 RESULTS
3.1 Eligible studies and characteristics
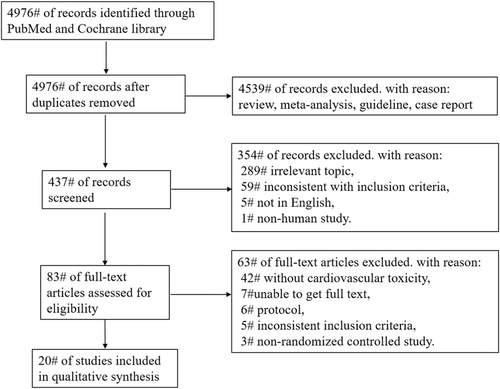
Overall, 4976 trials were obtained when searching the Cochrane Library and PubMed databases. After eliminating duplicate literature and carefully screening the titles, abstracts, and full text, 20 trials were included [7, 8, 12-29]. Figure 1 shows the search strategy and trial selection flow chart. We evaluated the quality of the included trials, and the results are shown in Figure 2. The details of the 20 trials are summarized in Table 1. We categorized the trials as (1) CTLA-4 inhibitors to placebo (five trials, 3553 patients), (2) CTLA-4 inhibitors combined with chemotherapy to chemotherapy alone (one trial, 1539 patients), (3) CTLA-4 inhibitors combined with PD-1/PD-L1 inhibitors to PD-1/PD-L1 inhibitors alone (11 trials, 3949 patients), or (4) CTLA-4 inhibitors combined with target agent to PD-1/PD-L1 inhibitors combined with target agent (three trials, 1314 patients). The most common tumor types in the trials were melanoma (five trials, 25%), NSCLC (three trials, 15%), small cell lung cancer (SCLC) (three trials, 15%), prostate cancer (two trials, 10%), malignant mesothelioma (two trials, 10%), urothelial carcinoma (one trial, 5%), gastroesophageal adenocarcinoma (one trial, 5%), pancreatic cancer (one trial, 5%), advanced or metastatic solid tumors (one trial, 5%), and BTC, ESCC, or HNSCC (one trial, 5%).
| Article | Cancer type | Treatment | Enrollment | All-grade cardiotoxicity | Grades 3–5 cardiotoxicity | All-grade hypertension | Grades 3–5 hypertension |
|---|---|---|---|---|---|---|---|
| CTLA-4 inhibitor vs. placebo | |||||||
| Maio 2017 | Malignant mesothelioma | Tremelimumab (10 mg/kg) | 382 | 16 | 11 | NA | NA |
| Placebo | 189 | 7 | 5 | NA | NA | ||
| Beer 2017 | Prostate cancer | Ipilimumab (10 mg/kg) | 399 | 2 | 2 | NA | NA |
| Placebo | 199 | 0 | 0 | NA | NA | ||
| Eggermont 2016 | Melanoma | Ipilimumab (10 mg/kg) | 475 | 1 | 1 | NA | NA |
| Placebo | 476 | 0 | 0 | NA | NA | ||
| Kwon 2014 | Prostate cancer | Ipilimumab (10 mg/kg) | 393 | 26 | 19 | 20 | 1 |
| Placebo | 396 | 13 | 7 | 13 | 1 | ||
| Ribas 2013 | Melanoma | Ipilimumab (10 mg/kg) | 325 | 1 | 1 | NA | NA |
| Placebo | 319 | 0 | 0 | NA | NA | ||
| CTLA-4 inhibitor + chemotherapy vs. chemotherapy | |||||||
| Tarhini 2019 | melanoma | Ipilimumab (3 mg/Kg) | 516 | 1 | 1 | NA | NA |
| Ipilimumab (10 mg/Kg) | 503 | 2 | 1 | NA | NA | ||
| HDI | 520 | 0 | 0 | NA | NA | ||
| CTLA-4 inhibitor + PD-1/PD-1 inhibitor vs. PD-1/PD-1 inhibitor | |||||||
| Paz-Ares 2022 | ES SCLC | Durvalumab (1500 mg) + tremelimumab (75 mg) + EP | 266 | 4 | 4 | ||
| Durvalumab (1500 mg) + EP | 256 | 3 | 3 | ||||
| Doki 2022 | BTC, ESCC, HNSCC | Tremelimumab (1 mg/kg) + durvalumab (10 mg/kg) | 124 | NA | NA | 1 | 0 |
| Durvalumab (10 mg/kg) | 116 | NA | NA | 3 | 0 | ||
| Goldman 2021 | ES-SCLC | Durvalumab (1500 mg) + tremelimumab (75 mg) + platinum-etoposide | 266 | NA | NA | 15 | 7 |
| Durvalumab (1500 mg) plus platinum-etoposide | 265 | 1 | 1 | 16 | 8 | ||
| Cascone 2021 | Metastatic NSCLC | Nivolumab (3 mg/kg) + ipilimumab (1 mg/kg) | 21 | 1 | 0 | NA | NA |
| Nivolumab (3 mg/kg) | 23 | 1 | 0 | NA | NA | ||
| Boyer 2021 | Metastatic NSCLC | Pembrolizumab (200 mg) + ipilimumab (1 mg/kg) | 282 | 7 | 6 | 7 | 7 |
| Pembrolizumab (200 mg + placebo | 281 | 8 | 7 | 4 | 4 | ||
| Zimmer 2020 | Melanoma | Nivolumab (1 mg/kg) + ipilimumab (3 mg/kg) | 55 | 2 | 0 | NA | NA |
| Nivolumab (3 mg/kg) | 56 | 1 | 0 | NA | NA | ||
| Powles 2020 | Urothelial carcinoma | Durvalumab (1500 mg) + tremelimumab (75 mg) | 340 | 0 | 0 | NA | NA |
| Durvalumab (1500 mg) | 345 | 4 | 3 | NA | NA | ||
| Planchard 2020 | Metastatic NSCLC | Durvalumab (20 mg/kg + tremelimumab (1 mg/kg) | 173 | NA | NA | 3 | 2 |
| Durvalumab (10 mg/kg) | 117 | NA | NA | 1 | 0 | ||
| Scherpereel 2019 | Malignant pleural mesothelioma | Nivolumab (3 mg/kg) + ipilimumab (1 mg/kg) | 61 | 1 | 1 | NA | NA |
| Nivolumab (3 mg/kg) | 63 | 1 | 1 | NA | NA | ||
| Hodi 2018 | Advanced melanoma | Nivolumab (1 mg/kg) + ipilimumab (3 mg/kg) | 313 | NA | NA | 4 | 1 |
| Nivolumab (3 mg/kg) | 313 | NA | NA | 5 | 2 | ||
| Antonia 2016 | SCLC | Nivolumab (3 mg/kg, 1 mg/kg) + ipilimumab (3 mg/kg, 1 mg/kg) | 115 | 1 | 1 | NA | NA |
| Nivolumab (3 mg/kg) | 98 | 1 | 1 | NA | NA | ||
| CTLA-4 inhibitor + target agent vs. PD-1/PD-1 inhibitor + target agent | |||||||
| Shitara 2022 | Gastro-esophageal adenocarcinoma | Nivolumab (1 mg/kg) + ipilimumab (3 mg/kg) | 403 | 2 | 2 | NA | NA |
| Nivolumab (1 mg/kg) + chemotherapy | 782 | 0 | 0 | NA | NA | ||
| Reiss 2022 | Pancreatic cancer | Niraparib (200 mg + ipilimumab (3 mg/kg) | 45 | NA | NA | 6 | 4 |
| Niraparib (200 mg) + nivolumab (240 mg) | 46 | NA | NA | 4 | 4 | ||
| Zamarin 2020 | Advanced or metastatic solid tumors | Mogamulizumab (1 mg/kg) + tremelimumab (10 mg/kg) | 19 | NA | NA | 1 | 1 |
| Mogamulizumab (1 mg/kg) + durvalumab (10 mg/kg) | 19 | NA | NA | 0 | 0 | ||
- Abbreviations: EP, etoposide plus either cisplatin or carboplatin; HDI, high-dose interferon alfa.
3.2 Incidence of cardiovascular adverse events
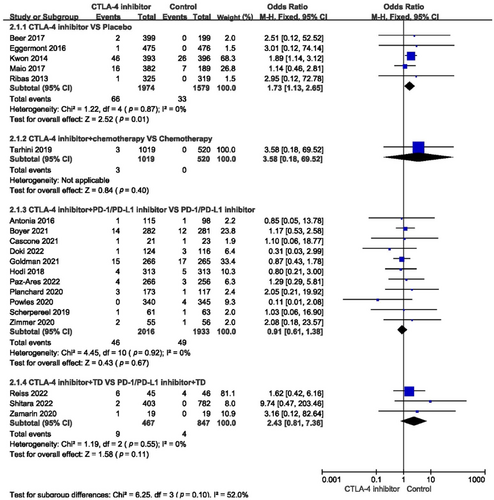
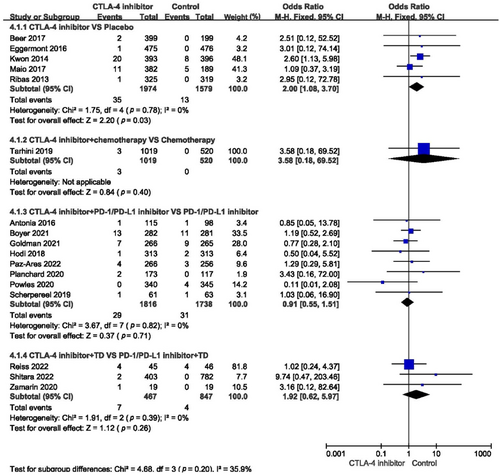
The incidence of myocardial infarction, cardiac failure, pericardial effusion, cardiac arrest, myocarditis, hypertension, and atrial fibrillation in the different treatments is shown in Table 2. Among all studies, the incidence of all-grade cardiovascular toxicity was 1.33 (95% confidence interval [CI]: 1.00–1.75, p = 0.05) (Figure 3). Subgroup analysis showed that compared with placebo, single-agent CTLA-4 inhibitors significantly affected the incidence of all-grade cardiovascular toxicity (odds ratio [OR] = 1.73, 95% CI: 1.13–2.65, p = 0.01) (Figure 4). When compared with chemotherapy alone, the combination with a CTLA-4 inhibitor did not influence the incidence of all-grade cardiovascular adverse events (OR = 3.58, 95% CI: 0.18–69.52, p = 0.40). Compared with using a PD-1/PD-L1 inhibitor or target agent alone, a CTLA-4 inhibitor combined with a PD-1/PD-L1 inhibitor or target agent did not impact the incidence of all-grade cardiovascular toxicity (OR = 0.91, 95% CI: 0.61–1.38, p = 0.67; OR = 2.43, 95% CI: 0.81–7.36, p = 0.11, respectively) (Figure 4).
| CTLA-4 inhibitor (N = 1974, 55.56%) | Placebo (N = 1579, 44.44%) | CTLA-4 inhibitor (N = 1019, 66.21%) | Chemotherapy (N = 520, 33.79%) | CTLA-4 inhibitor + PD-1/PD-1 inhibitor (N = 2016, 51.05%) | PD-1/PD-1 inhibitor (N = 1933, 48.95%) | CTLA-4 inhibitor + TA (N = 467, 35.54%) | PD-1/PD-1 inhibitor + TA (N = 847, 64.46%) | |
|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | 7 (0.35) | 3 (0.19) | NA | NA | NA | NA | NA | NA |
| Cardiac failure | 5 (0.25) | 5 (0.25) | NA | NA | 1 (0.05) | NA | 2 (0.43) | NA |
| Pericardial effusion | 15 (0.76) | 6 (0.39) | NA | NA | 4 (0.20) | 9 (0.47) | NA | NA |
| Cardiac arrest | 7 (0.35) | 2 (0.13) | 2 (0.20) | NA | 1 (0.05) | 3 (0.16) | NA | NA |
| Myocarditis | 1 (0.05) | NA | 1 (0.10) | NA | 4 (0.20) | 3 (0.16) | NA | NA |
| Hypertension | 20 (1.01) | 13 (0.82) | NA | NA | 30 (1.50) | 29 (1.50) | 7 (1.50) | 4 (0.47) |
| Atrial fibrillation | 5 (0.25) | 4 (0.25) | NA | NA | 3 (0.15) | 3 (0.16) | NA | NA |
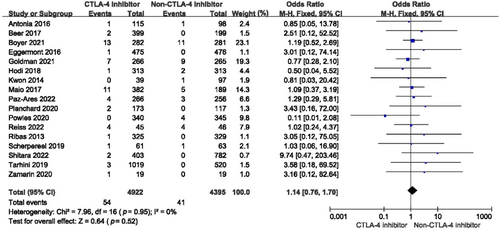
For grades 3–5 cardiovascular toxicity, the incidence among all studies did not increase (OR = 1.14, 95% CI: 0.76–1.70, p = 0.52) (Figure 5). Compared with placebo, the risk of severe cardiovascular toxicity when using a single-agent CTLA-4 inhibitor increased dramatically (OR = 2.00, 95% CI: 1.08–3.70, p = 0.03) (Figure 6). When a CTLA-4 inhibitor was combined with chemotherapy or PD-1/PD-L1 inhibitor or target agent, the risk of grades 3–5 cardiovascular toxicity did not increase (OR = 3.58, 95% CI: 0.18–69.52, p = 0.40; OR = 0.91, 95% CI: 0.55–1.51, p = 0.71; OR = 1.92, 95% CI: 0.62–5.97, p = 0.26, respectively) (Figure 6).
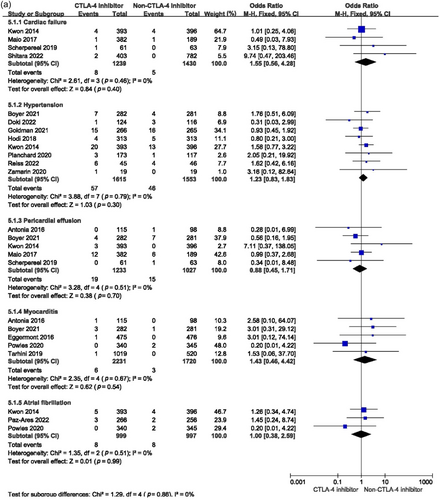
An analysis was also conducted to estimate the risks of myocardial infarction, cardiac failure, pericardial effusion, cardiac arrest, myocarditis, hypertension, and atrial fibrillation. CTLA-4 inhibitors did not statistically influence the incidence of these events compared with placebo, chemotherapy, another ICI, or target agent (Figure 7a,b). Subgroup analysis found that the risks of severe cardiac failure (OR = 1.85, 95% CI: 0.63–5.41, p = 0.26), hypertension (OR = 1.24, 95% CI: 0.67–2.27, p = 0.49), pericardial effusion (OR = 1.26, 95% CI: 0.50–3.18, p = 0.62), myocarditis (OR = 1.29, 95% CI: 0.36–4.66, p = 0.70), and atrial fibrillation (OR = 2.24, 95% CI: 0.69–7.28, p = 0.18) were much higher among patients exposed to CTLA-4 inhibitor. However, there were few patients involved, so these differences were not statistically significant.

4 DISCUSSION
This study found that the incidence of all-grade cardiovascular toxicity was significantly increased in the patients exposed to CTLA-4 inhibitors. In contrast, the risk of grades 3–5 cardiovascular toxicity did not increase. Subgroup analysis showed that the use of a single-agent CTLA-4 inhibitor significantly increased the incidence of all-grade cardiovascular toxicity and severe cardiovascular toxicity compared with placebo. For combination regimens, the incidence of cardiac and vascular adverse events among patients exposed to CTLA-4 inhibitors did not change. Additional analysis of individual cardiovascular adverse events suggested that CTLA-4 inhibitors may be associated with an increased risk of severe cardiovascular adverse events.
As described above, we found that a single-agent CTLA-4 inhibitor observably increased the incidence of cardiovascular toxicity. Ipilimumab was the first ICI used to treat metastatic melanoma [30]. It was reported that all-grade adverse events could occur in 60% of patients treated with ipilimumab, while all severe adverse events could occur in 10%–30% of these individuals [31]. Tremelimumab is another CTLA-4 inhibitor, which has been in clinical use for a shorter time than ipilimumab. The risk of cardiovascular adverse events was reportedly 4.19% when single-agent tremelimumab was used to treat malignant mesothelioma [21]. When tremelimumab was combined with durvalumab, the incidence was 5.64% [18]. Recently, Rubio-Infante et al. [32] reported that the incidence of cardiac adverse events with a CTLA-4 inhibitor was 3.6% among 3764 treated patients. Xavier et al. [33] also reported that the incidence of all-grade cardiovascular toxicity with CTLA-4 inhibitors was 8.33% and 5.48% for severe cardiovascular adverse events, much higher than the 1% reported in most trials. Among the cardiovascular adverse events caused by CTLA-4 inhibitors, severe events accounted for the majority, which is consistent with our conclusions in this study. When comparing the results of the Rubio-Infante et al. [32] and Xavier et al. [33] studies, CTLA-4 inhibitors were also found to increase the incidence of vascular adverse events.
Patients using higher doses (10 mg/kg) of ipilimumab have been reported to have a higher risk of adverse events than those using lower doses (3 mg/kg) [34]. Tumor responses have also been associated with drug dose. With this, additional questions arise: whether adverse events indicate better tumor outcomes when patients use ICIs [35]. According to work by Downey et al. [36], among the patients with metastatic melanoma who received ipilimumab, those who developed immune-related adverse events were associated with better antitumor outcomes. Attia et al. [37] showed that patients with severe autoimmune toxicity experienced better clinical responses compared with those with no autoimmune toxicity. These studies showed that the responses of tumors treated with an anti-CTLA-4 antibody correlated with immune-related adverse events. Fortunately, most adverse events were grade 1 or 2, with almost all being reversible with administration of high-dose steroids [37].
However, the mechanism by which ICIs can induce cardiovascular toxicity remains unclear. Because of the association between drug dose and immune-related adverse events, we hypothesized that disruption of peripheral immune system tolerance may be the underlying mechanism. One case was reported that involved abatacept, a CTLA-4 agonist that has been approved for use in patients with rheumatic diseases, leading to the resolution of myocarditis that was induced by an ICI [38]. CTLA-4 inhibitors may cause cardiovascular adverse events through two mechanisms. First, tumor cells and cardiomyocytes share some muscle-specific antigens, which can trigger cross-reactivity with T cells [39, 40]. Skeletal muscle cells may also share the same muscle-specific antigens, as the first symptom of most patients with immune myocarditis is ptosis. Additionally, immune cell infiltration can be observed in the skeletal muscle of the eyelid. In the affected hearts of PD-1 (−/−) BALB/c mice, complement C3 and immunoglobulin G, specific antibodies to cardiac troponin I, were diffused on the surface of cardiomyocytes [41]. Second, CTLA-4 plays a vital role in normal immune function. Therefore, ICI-mediated inhibition may cause cardiac cells to be more susceptible to injury [42]. Once CTLA-4 on the T cell surface competitively binds to B7, activated T cell proliferation is limited. Thus, the injured area can be protected, reducing unnecessary damage [2]. When CTLA-4 inhibitors interact with CTLA-4, T cell proliferation is not limited, causing excessive production of inflammatory factors in the injured area and immune damage.
Since 2015, the combination of CTLA-4 inhibitors and PD-1/PD-L1 inhibitors has shown excellent efficacy in advanced melanoma [43], renal cell carcinoma [44], microsatellite instability-high malignancy [45], SCLC [12], and NSCLC. However, the risk of toxicity was also higher when the two ICIs were used together [46]. A recent meta-analysis showed that currently used PD-1, PD-L1, and CTLA-4 inhibitors exhibited similar incidence rates of cardiac and vascular toxicity, with 7.59% (95% CI: 5.31%–10.22%), 7.69% (95% CI: 3.88%–12.60%), and 8.33% (95% CI: 3.40%–15.08%), respectively. However, the incidence of combination therapy is higher (12.45%, 95% CI: 4.99%–22.56%) [33]. Another meta-analysis also showed that compared with single-agent ICI, dual immunotherapy demonstrated a higher incidence of cardiovascular adverse events (3.1% and 5.8%, respectively), which suggested that the type of ICI was relevant [32]. Because of this, closer observation is warranted in patients who are receiving dual immunotherapy. In this study, we also compared the risk of a CTLA-4 inhibitor combined with a PD-1/PD-L1 inhibitor to that with a PD-1/PD-L1 inhibitor alone. However, the incidence of cardiovascular toxicity was not increased with this combination, which was consistent with other trials [47, 48]. There are several possible reasons why our results showed that combination therapy did not result in higher cardiovascular toxicity compared with monotherapy. First, patients in both groups may have received therapies other than ICIs as part of their treatment regimens. Second, because of the inclusion and exclusion criteria, we did not include all the trials that compared the combination of two ICIs to ICI monotherapy. Third, patients receiving PD-1 inhibitors may have a higher risk of toxic cardiovascular events than those taking CTLA-4 inhibitors. Rubio-Infante et al. [32] performed a pharmacovigilance database analysis of the VigiAccess database, finding that at least 69.4% of cardiac disorders were associated with nivolumab and pembrolizumab treatment and 20% with ipilimumab and tremelimumab treatment. This would also result in no difference in the incidence of cardiovascular toxicity with the combination of the two ICI types compared with using a PD-1 inhibitor alone.
This study has some limitations. The included trials covered a wide range of cancer types and treatment regimens that also varied in the control group, which could potentially create heterogeneity. Additionally, certain subgroup analyses had few included studies. In the future, larger clinical studies need to be included to clarify the cardiotoxicity of CTLA-4 inhibitors.
5 CONCLUSION
In summary, we found that CTLA-4 inhibitors could significantly increase the incidence of cardiovascular toxicity in patients. Monitoring toxic cardiovascular events during ICI treatment is not currently routinely recommended by guidelines. From the results of our analysis, we recommend performing a routine evaluation of cardiac structure and function during ICI use. How to reduce the cardiotoxicity associated with CTLA-4 inhibitors is an urgent problem that should be addressed.
AUTHOR CONTRIBUTIONS
Huiyi Liu: Writing—original draft (equal). Lu Fu: Methodology (equal). Shuyu Jin: Methodology (equal). Xingdong Ye: Acquisition of data. Yanlin Chen: Formal analysis (equal). Sijia Pu: Funding acquisition (equal). Yumei Xue: Writing—review & editing.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data used to support the findings of this study are included in the article.




