Advanced flexible brain-computer interfaces and devices for the exploration of neural dynamics
Pancheng Zhu and Mengxia Yu contributed equally.
Abstract
The rapid advancement of flexible neural interfaces and devices is revolutionizing our ability to explore the neural foundations of consciousness, intelligence, and behavior. Cutting-edge developments in materials science and system-level integration are significantly enhancing the spatiotemporal resolution of neural signal acquisition and modulation, paving the way for next-generation brain-computer interfaces. These technologies enable unprecedented investigations into the causal relationships between neural dynamics and behaviors in freely moving subjects, offering new insights into various neurocognitive domains. The integration of artificial intelligence and brain organoids with neuroscience research promises to further decode complex neural signals, deepening our understanding of multilevel neural dynamics. Beyond their scientific implications, these innovations also offer transformative possibilities for the diagnosis, treatment, and management of neurological and psychiatric disorders. This perspective paper examines how flexible neural interfaces overcome the limitations of traditional neurotechnology, their potential impact on neural research, and their promising applications in treating neurological and psychiatric disorders, while also considering the ethical implications and future challenges in this rapidly evolving field.
Key points
What is already known about this topic?
-
Current understanding indicates that complex nervous system dynamics stem from intricate interactions between neurons, their environments, and brain networks. To further explore these neural dynamics, advanced neural interfaces and devices are needed to address limitations in current neurotechnologies.
What does this study add?
-
This perspective paper compiles recent advances in flexible neural interface technologies, highlighting the synergy of material innovation and device-level integration. This integration supports research on free-behaving animals, enhancing our understanding of neurocognitive processes. Advances in artificial intelligence and brain organoid use show promise for decoding neural signals, while transient materials and devices offer new strategies for treating neurological disorders and neurorehabilitation.
1 INTRODUCTION
Understanding brain activity requires unraveling how neurons, the fundamental units of the nervous system, orchestrate essential functions such as perception, cognition, consciousness, and behavior.1 Human actions emerge from intricate neural processes that integrate inputs from both internal states and external stimuli through sophisticated biological algorithms. As illustrated in Figure 1, our current understanding suggests that the complex choreography of the nervous system arises from the delicate interplay between individual neurons, their microscopic environment, and extensive brain networks.11
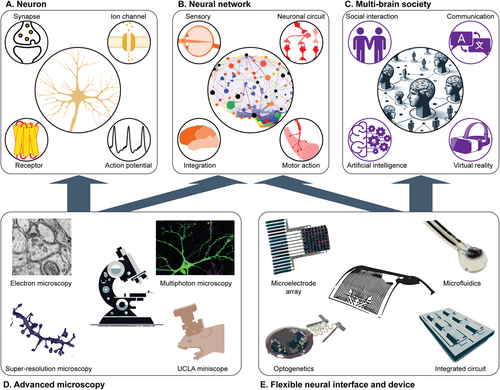
Hierarchical explorations of neural dynamics with advanced neurotechnology. (A) Molecular and cellular explorations of the function of individual neurons. (B) Explorations of neural circuit mechanisms underlying cognitive functions. (C) In the modern era, explorations of multi-brain network algorithms involve social interactions, communication, and advanced technologies. (D) Examples of major microscopy methodologies in the investigation of individual neurons and their networks. Images are reproduced with the permission of refs.2-5 (E) Examples of flexible neural interfaces and their device-level integration for monitoring neural dynamics and modulating neural activities via electrical, optical, and pharmacological methods. Images are reproduced with the permission of refs.6-10
1.1 Multilevel explorations of neural dynamics
At the cellular level, our understanding of neurons has been largely shaped by genetic and molecular perspectives; neurons are classified by their transcriptional and translational profiles.12 Meanwhile, ion channels, neurotransmitters, receptors, and intracellular cascades all play crucial roles in neural transmission, establishing neurons as the fundamental components of a complex communication network. For example, ion channels open in response to electrochemical events in the extracellular environment, generating ion movements and electrical signals that propagate along the neuron. In response to these electrical signals, neurotransmitters are released at the synapse, binding to receptors on downstream neurons to transmit information within the neural network13 (Figure 1A). These fundamental insights, primarily gained through traditional molecular and electrophysiological approaches, provide the basis for modern techniques, such as single-cell spatial transcriptomics and multi-omics, which allow us to address more fundamental neurobiological questions.14, 15 Recent discoveries include the identification of distinct neuronal subpopulations linked to specific functional circuits and brain layers,16 the mapping of neuronal differentiation trajectories and gene expression dynamics occurring during neural stem cell maturation,17 and the uncovering of molecular and cellular signatures of neurodegenerative and psychiatric disorders.18
Investigations at the level of diffuse communication have elucidated how synapses, dendritic integration, and axonal pathways connect neurons and converge them into networks. These networks form and bridge distinct brain regions, enabling the brain to process environmental stimuli, supporting sensory perceptions, motor functions, and the higher cognitive processes necessary for interaction with the external world19 (Figure 1B). Neural tracing and microscopy have provided foundational approaches for addressing various questions at this level, from uncovering topographic maps in sensory systems20 to discovering brain-wide connectomes.21 In parallel, techniques like optogenetics allow for neuronal manipulation, leading to the identification and dissection of specific excitatory and inhibitory circuits responsible for various behaviors.1
At the individual and societal levels, neural dynamics extend from single to multiple brains during communication and cooperation, further shaping both personal and interpersonal behaviors. These interactions form the foundation for societal and ecological structures.22 Many distinctive cognitive features of mammals, such as mentalizing, attachment, and relationships, can only be fully understood within a social context.23 In the contemporary era, the integration of technology and artificial intelligence (AI) with human activities introduces new dimensions to our understanding of behavior and consciousness (Figure 1C). These technologies have revolutionized human social features by transforming how we communicate, form relationships, and interact across both digital and physical environments; these changes further complicate the decoding of socio-cognitive neural responses as our experiences and interactions increasingly transcend physical boundaries.
Despite its remarkable achievements, many interesting questions in neuroscience remain unclear. For instance, how neural activities generate behavior in real time, what the neural dynamics behind complex thoughts and intentions are, and whether modern technologies can create new sensory modalities in the brain. Answering these questions will require capabilities beyond conventional neuroscience methodologies. Novel technologies will be necessary to explore, understand, and interact with the brain in currently unattainable ways.
Neural interfaces, which first emerged in the early 20th century, have established a communication pathway between the brain and external platforms by allowing interaction with electrophysiological signals in proximity to neurons.24 In the 21st century, advances in material science and micro/nano-fabrication have led to the development of flexible and biocompatible neural interfaces. These innovations support advanced neuroprosthetics, closed-loop neural stimulation systems, and AI-based neural signal decoding. These capabilities effectively bridge the gap between laboratory discoveries and clinical applications.
1.2 Limitations of current neurotechnology
The goal of neurotechnology is to access microscopic neural details while maintaining a broader perspective on entire networks and behaviors. For example, high-resolution electron microscopy satisfies some aspects of this requirement by providing detailed neural wiring diagrams from anatomically dissected brain slices.2 These diagrams reveal the positions of individual neurons and their synaptic connections, offering a direct observation of the neural circuits (Figure 1D). One representative work in this area mapped the whole neural circuitry of an adult drosophila melanogaster by analyzing 21 million images, comprising 106 terabytes of raw data.25 However, this method becomes impractical when applied to species with larger brain volumes (e.g., rodents, reptiles, and primates) due to the extensive labor required for slicing, imaging, and complex data processing. Moreover, these dissections of neural circuits cannot be performed on live animals, thus preventing the exploration of neural mechanisms that are relevant to dynamic activities.
Understanding the neural bases that shape behaviors and consciousness requires the exploration of functional dynamics, both of individual neurons and of their activity within networks of living animals. To address this challenge, research and engineering efforts leverage advanced fabrication techniques from the semiconductor industry to produce penetrating probes and shanks to monitor brain activity. Representative achievements include the development of silicon probes with densely packed, individually accessible electrode arrays capable of recording neural activity in large tissue volumes with cellular-scale spatiotemporal resolution.26 An example is the Neuropixels 2.0 probe, which enables chronic neural recordings in small mammals at over 5000 sites simultaneously.27 Additionally, the integration of individual probes into arrays, commonly known as Utah Arrays, enhances the monitoring of neural activity in the lateral dimension. One study investigated whether intracortical microstimulation (ICMS) could provide artificial somatosensory feedback through brain-computer interfaces (BCIs) for tetraplegic individuals. Using a Utah multi-electrode array, researchers recorded neural responses to ICMS in rhesus macaques. They found that ICMS led to both trans-synaptically evoked activity and suppression of neural activity, indicating that artificial biomimetic stimulation patterns can enhance artificial sensory feedback.28 Extensive live-animal studies using these technologies have facilitated major advances relating to the mammalian nervous system, ranging from insights into neural representation of sensory characteristics in the visual cortex29 to real-time transformation of cortical signals to control complex prosthetic robots.30
The combination of neuron-labeling technology with light delivery and collection tools has enabled the modulation and detection of cell-specific activity. This has allowed the investigation of causal aspects of neural circuit function in relation to certain behaviors. Since early studies using the channelrhodopsin ChR2 to control orexin neurons,31 optogenetics has facilitated the discovery of neural circuits governing behaviors in various cognitive domains (e.g., social,32 sleep,33 learning,34 and affect35). Moreover, advances in ever-improving genetically encoded calcium indicators and neurotransmitter sensors have transformed optical methodologies for the study of neural function in living organisms. For instance, fluorescent imaging techniques have enabled the mapping of a cortical connectivity atlas in the mouse neocortex, revealing that the entire cortex is organized into subnetworks that exhibit distinct topologies and interact through specific cortical pathways.36
However, each of the above relies on rigid substrate materials and complex tethered wirings that limit their application in animal studies that require free motion over extended periods. Moreover, these bulky systems disturb the social behavior among groups of animals; this prevents the study of social interactions, which is crucial for exploring multi-brain networks. To address these limitations, the development of flexible neural interfaces and device-level integration is required (Figure 1E).
2 ADVANCES IN DETECTION AND MODULATION OF NEURAL ACTIVITY
2.1 Development of flexible neural interface
The detection and modulation of neural activity require technologies capable of both capturing signals that reflect the system dynamics and interacting with the system itself. Advances in material science and interface technology have produced flexible biocompatible interfaces that reduce immediate implantation trauma and chronic foreign-body responses, allowing studies to be performed in live animals over extended periods. One strategy focuses on developing new materials to soften the interfaces to conform to the complex and delicate structures of the brain, spinal cord, and peripheral nerves, reducing tissue damage and inflammation for long-term operation. Representative achievements include the development of hydrogels,37 conductive polymers,38 silicone,39 and nanomaterial composites.40 For example, the Flex2Chip, an ultra-conformable thin-film electrode array that self-assembles into silicon microelectrode arrays, enables multi-thousand channel counts at the millimeter scale. It has been used to successfully measure extracellular action potentials and resolve micrometer-scale seizure propagation in epileptic mice, revealing non-constant propagation trajectories in the absence of epilepsy.39
Based on the development of these compliant materials, another strategy exploits high-resolution fabrication techniques from the semiconductor industry to produce densely packed arrays of cellular- or subcellular-scale electrodes and semiconductor components, which can then be integrated with mechanically compliant substrates in the form of thin polymetric sheets or penetration probes.41 The resulting recordings of local field potentials in animal models provide insights into the correlation between neural activities and behavioral states. Alternatively, the use of micro-sized light-emitting diodes and photodiodes (∼10–300 μm), combined with fluorescent and genetic labeling technologies, enables cell-type and region-specific modulation and monitoring of neural activities.42 This extends the scope of neuroscience studies beyond what is possible with electrical methods alone.
Extensive experimental trials using these neural interfaces have shaped our understanding of the operational principles of neural circuits, ranging from the local excitation/inhibition of isolated cell populations to signal transmission across many distantly related neuronal subgroups and brain regions.36 For example, utilizing in situ electro-sequencing—combining flexible bioelectronics with in situ RNA sequencing—researchers were able to stably map millisecond-timescale electrical activity and profile single-cell gene expression from the same cells across intact biological networks. This technique facilitates the discovery of cell types and genetic expressions responsible for electrophysiological function and dysfunction.43
2.2 Progress in device-level integration of flexible neural interfaces
For studies focusing on social interactions and multi-brain networks, device-level integration of these flexible neural interfaces is crucial. Integrated systems allow for the removal of bulky terminal equipment and tether connections—typically necessary for power and signal transmission—enabling studies to be conducted in truly free-behaving animals. The recent development of flexible printed circuit boards and chips has enabled the integration of wireless signal transmission (i.e., near-field communication and Bluetooth) and power (e.g., batteries, inductive charging, and ultrasound) systems with flexible neural interfaces. The resulting devices have miniaturized implantable forms enabling applications in free-behaving animals for the study of relationships between neural activities and outcome behaviors. Representative achievements include real-time optogenetic modulation of rodents' social behaviors,6 and wireless recordings of neuro-physical activities in the form of electroencephalogram (EEG),44 electromyography (EMG),44 or behavioral ethograms (MA).45 In one study, a fully implantable subdermal wireless optogenetic device offered real-time user programmability over multiple stimulating light sources. Researchers were able to induce artificial interbrain neuronal synchrony and desynchrony in the medial prefrontal cortex between paired mice to modulate their social interactions in real-time.6
3 CHALLENGES IN FLEXIBLE INTERFACE AND DEVICE TECHNOLOGY
Despite these remarkable advances in neural interfaces and system-level integrations, significant impediments remain in the way of further exploration. From our perspective, the primary challenge is that the existing neural devices and interfaces used in live animals do not achieve the resolution and precision offered by anatomical- or neuro-labeling-facilitated microscopy. For example, although the size of the electrodes and optoelectronic components used in these advanced devices reach the cellular scale, their positions relative to individual neurons after implantation are usually poorly defined. Consequently, analysis is limited to regional brain activity as the signals collected represent a summation from multiple neurons of uncertain types and origins. Identifying the precise locations of neurons and tracing their signal transmission are essential steps yet to be achieved in vivo animal studies.
Meanwhile, despite the advent of flexible forms, the implantation of neural interfaces still leads to local physical damage to neuronal projections and chronic inflammation. This is particularly true for penetration probes. The disturbances surrounding implantation sites can vary substantially between individual animals and surgical implantations. Such physical impacts compromise the health status of the subjects and introduce uncontrollable variables that confound the experimental results. These limitations collectively prevent advanced neural interfaces and devices from clinical application. For example, the BCI technology presented by Neuralink is currently prevented from reaching clinical trials due to the uncertain damage and effects on the brain tissue from invasive electrode implantation.
4 FUTURE DIRECTIONS
We envision the development of new materials, device fabrication technologies, and signal processing methods, especially in the areas outlined below. Such advances will address the current challenges and promote the advancement of neural interfaces and devices for future neuroscience studies (Figure 2).
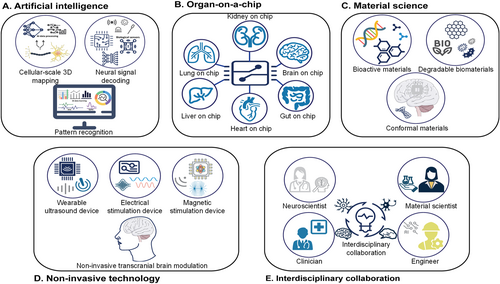
Future directions for promoting the development of next-generation neural interfaces and devices for advanced neuroscience study. (A) Artificial intelligence techniques for 3D mapping of neurons, including deep learning for signal decoding and pattern recognition. (B) Organ-on-a-chip technology. (C) Technological improvements in material science. (D) Non-invasive brain stimulation techniques such as ultrasound, electrical currents, and electromagnetic field stimulation. (E) Interdisciplinary collaboration among neuroscientists, engineers, material scientists, and clinicians in the field of neural interface and device technology.
4.1 Artificial intelligence
AI-related data processing and interpretation has great potential to achieve cellular-scale three-dimensional (3D) mapping of neurons and could enable the tracing of synaptic transmission from signals acquired from versatile biological sensors (electrical, optical, pharmacological). AI data learning applied to large datasets has been shown to be capable of differentiating and correlating factors affecting signals.46 We envision that applying deep learning methods will contribute to the decoding of neural signals and the identification of specific neural patterns associated with different mental states, movements, or sensory perceptions. Challenges in integrating AI technologies with neural interfaces include the need for real-time processing and reliable interpretation of neural signals as well as the development of robust algorithms that can adapt to individual variations (Figure 2A).
4.2 Organ-on-a-chip technology
Advances in organ-on-a-chip technology provide an in vitro platform that mimics the essential properties of target biological systems.47 Integrating multimodal sensors into such a system, with selective regional cultivation of neurons, will further enable researchers to explore synaptic transmission relating to the relative distributions of neurons, the environment, and stimuli. One pioneering work explored the nonlinear dynamics and fading memory properties of the neural networks in a brain organoid using AI-facilitated adaptive reservoir computation.48 Another work modeled sporadic Alzheimer's disease (sAD) using human induced pluripotent stem cell-derived 3D brain organoids exposed to human serum. The organoids exhibited AD-like pathologies, including amyloid beta aggregates, phosphorylated tau, synaptic loss, and impaired neural networks. Such a model offers a powerful platform for both mechanistic study and therapeutic developments in the future. The application of organ-on-a-chip platforms and organoids removes the complexity of animal studies, enabling the exploration of neural networks in a standardized and precisely controlled manner (Figure 2B).
4.3 Material science and engineering
Despite the inevitable physical damage and inflammation caused by implanted biomedical devices, particularly in deep brain areas, continued technological improvements in material science and device fabrication minimize these impacts. One promising technique that has gained traction in recent years is the use of bioactive materials, such as bioceramics49 and bioactive hydrogels,50 which can promote tissue growth and support healing by releasing ions that can stimulate cellular activity; examples include calcium, phosphate, and silica. For example, a bioglass/oxidized sodium alginate composite hydrogel, with adipic acid dihydrazide-modified γ-polyglutamic acid as a cross-linker, exhibited dual adhesive and bioactive properties. It enhanced tissue bonding, promoted vascularization, and accelerated tissue regeneration, showing promising potential for wound-healing applications.50 Additionally, the application of transient materials for functional components will enable programmable degradation of the device in the biological environment after implantation, eliminating the need to surgically remove these devices. This is of particular importance for future clinical applications (Figure 2C). One representative study used combinations of bioresorbable materials including tungsten-coated magnesium (W/Mg), doped monocrystalline silicon nanomembrane (Si NM), a film of a poly(lactide-co-glycolide) (PLGA), and candelilla wax to assemble a battery-free cardiac pacemaker. The device relied exclusively on materials that resorbed when exposed to biofluids in a time-controlled manner via metabolic action and hydrolysis.51 Another study developed biodegradable batteries for innovative medical treatments. The fully biodegradable primary Zn–Mo battery with anode electrodes was based on sintered nanoparticles, with normal saline or gelatin hydrogel serving as the electrolyte. This battery had the advantages of full degradation, prolonged operational lifetime (up to ∼19 days), and desirable output voltage (up to ∼0.7 V) and energy capacity (>1500 μWh) compared with previously reported primary Zn biobatteries.52
4.4 Non-invasive methods for neural activity monitoring and modulation
A unique strategy exploits non-invasive methods such as ultrasound and electromagnetic fields to stimulate the brain and collect corresponding signals from outside the skull; this significantly reduces the complexities and risks of surgical implantation compared to invasive methods.53 In one representative work, Jog et al. applied serial transcranial direct current stimulation (tDCS) to the left dorso-lateral prefrontal cortex (DLPFC) in patients with depression. They observed significant treatment-related gray matter changes with active high-definition (HD) tDCS relative to sham tDCS within the left DLPFC stimulation target, demonstrating that serial HD-tDCS leads to neurostructural changes at a predetermined brain target in depression.54 Another work introduced a novel clinical sonication technique using single ultrashort ultrasound pulses for brain modulation, referred to as transcranial pulse stimulation (TPS). Clinical trials with Alzheimer's patients using TPS demonstrated high treatment tolerability, significant neuropsychological improvements lasting up to 3 months, and correlated memory network upregulation.55 These achievements support the potential for broad neuroscientific applications and further clinical studies (Figure 2D).
4.5 Interdisciplinary collaboration
Future development of neurotechnology requires collective efforts from various fields including but not limited to neuroscience, material science, mechanical engineering, interface engineering, electrical engineering, biomedical engineering, and pharmacology. Collaboration between scientists with different backgrounds is imperative. The science community should encourage academic communication by providing opportunities for interdisciplinary education, training, conferences, and funding (Figure 2E). Collaboration between academia and industry should also be encouraged to promote clinical trials and eventually translate neurotechnologies into products that benefit human society. However, regulations on data privacy and experimental scope must be strictly imposed and enforced to ensure that scientific explorations follow ethical standards, preventing the malicious application of neurotechnology that disturbs the social structure for economic profit.
5 CONCLUSIONS AND PERSPECTIVE
Humanity's curiosity has driven the development of neuroscience research, leading to attempts to decipher the mechanisms behind neural dynamics and explain how we feel, sense, think, and behave. Today, the urgent need to apply this knowledge to treat neuropsychiatric disorders, such as autism and depression, continues to motivate exploration in this field. The development of flexible neural interfaces and devices that involve multimodal sensing and modulation of neural activities, along with wireless charging and communication technologies, has helped overcome the constraints of traditional anatomical methods. These innovations extend the research possibilities to freely behaving animals, expanding our understanding of neural dynamics ranging from synaptic signal transmissions between individual neurons to social behaviors involving multi-brain networks.
Further advances in material science, device/system-level integrative technologies, and AI-enhanced data analysis are expected to further refine neural interfaces and devices. The improvements are aimed at enhancing resolution and accuracy in tracing neural signals and expanding their applicability in clinical settings. By doing so, neuroscientists promise to deliver more profound insights into neural dynamics and offer transformative solutions that could shape the future of mental health. Such methodological evolution underscores the interconnected nature of our discoveries, emphasizing their significant implications for understanding humanity, directing therapeutic interventions, and shaping the future evolution of society.
AUTHOR CONTRIBUTIONS
Pancheng Zhu: Investigation; writing—original draft; writing—review & editing. Mengxia Yu: Investigation; writing—original draft; writing—review & editing. Mingzheng Wu: Conceptualization; investigation; supervision; writing—original draft; writing—review & editing. Yiyuan Yang: Conceptualization; investigation; supervision; writing - original draft; writing—review & editing.
ACKNOWLEDGMENTS
This work is supported by the National University of Singapore Presidential Young Professorship Award (to A-0010009-00-00 Yiyuan Yang).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




