Oxidative stress in hydrocephalus: A new potential therapeutic target
Jie Zhao and Zhi Tang contributed equally to this work and share the first authorship.
Abstract
Hydrocephalus is an abnormal accumulation of cerebrospinal fluid within the skull for several reasons, such as cerebrospinal fluid overproduction, circulatory obstruction, and malabsorption. Excess fluid causes the ventricular system and subarachnoid space to enlarge due to the squeezing of cerebrospinal fluid. Hydrocephalus is clinically manifested by increased intracranial pressure and impaired brain function. It is a neurological disease with a variety of complications that affect the body and require long-term and continuous treatment; however, current treatment methods are relatively limited, whether medical or surgical. Studies have shown that oxidative stress plays an important role in the formation and development of hydrocephalus, but it has not been systematically reviewed in current studies. In this paper, oxidative stress in hydrocephalus formation and its potential role were systematically reviewed, including the mechanism of oxidative stress, related signaling pathways, and pathological changes in oxidative stress formation. The purpose of this paper is to illustrate the possibility of oxidative stress as a new therapeutic target of hydrocephalus treatment, expecting that it will be helpful for future research.
Key points
What is known about this topic?
-
Hydrocephalus refers to diseases characterized by physiological disorders of cerebrospinal fluid, its abnormal accumulation, and hemodynamic changes. At present, no mechanism is clearly defined. Many recent studies have shown that the pathological changes of secondary hydrocephalus, especially cerebral hemorrhage, infection, and post-traumatic hydrocephalus, are related to oxidative stress. Current treatment of hydrocephalus is mainly surgical, followed by supplementary drugs or other treatments, but the outcomes are unsatisfactory.
What does this study add?
-
This paper reviews the relationship between oxidative stress and hydrocephalus, including the causes of oxidative stress in hydrocephalus and the pathological damage it causes, the relevant signaling pathways, and antioxidants with therapeutic potential. Thus, this review should make a positive contribution to future research on hydrocephalus.
Abbreviations
-
- A1M
-
- α1-microglobulin
-
- AQP-4
-
- aquaporin-4
-
- BBB
-
- blood-brain barrier
-
- CBF
-
- cerebral blood flow
-
- CSF
-
- cerebrospinal fluid
-
- EBI
-
- early brain injury
-
- ETC
-
- electron transport chain
-
- Glu
-
- glutamate
-
- GMH-IVH
-
- Germinal stromal ventricular hemorrhage
-
- GSH
-
- glutathione
-
- GSH-Px
-
- glutathione peroxidase
-
- GSH-Rd
-
- glutathione reductase
-
- ICP
-
- intracranial pressure
-
- MCI-186
-
- 3-metil-1-fenil-2-pyrazolin-5-ona
-
- NO
-
- nitric oxide
-
- NPCs
-
- neural progenitor cells
-
- RNS
-
- reactive nitrogen species
-
- ROS
-
- reactive oxygen species
-
- SAH
-
- subarachnoid hemorrhage
-
- SAS
-
- subarachnoid space
-
- SOD2
-
- superoxide dismutase 2
1 INTRODUCTION
Hydrocephalus refers to a class of diseases characterized by physiological disorders of cerebrospinal fluid, its abnormal accumulation, and hemodynamic changes.1 It can lead to increased intracranial pressure (ICP), often resulting in decreased cerebral blood flow and worsening of the condition.2 The most common classifications of hydrocephalus are obstructive hydrocephalus and traffic hydrocephalus. The former is caused by abnormal accumulation of cerebrospinal fluid due to interruption of flow from the ventricles to the subarachnoid space and is commonly associated with arachnoid cysts, aqueductal atresia or stenosis, median or interventricular foramen hypoplasia, Chiari malformation, or craniopharyngioma.3 The latter is related to cerebrospinal fluid's interrupted absorption or blocked loop from the subarachnoid space to the venous circulation.4
A growing body of research suggests that the development of some hydrocephalus is linked to several genetic factors. Mutated genes can lead to abnormalities in the development of the brain and nerves, which may impact the normal circulation of cerebrospinal fluid.5 Recent studies have revealed that congenital hydrocephalus is closely associated with genes regulating neural stem cell fate.6 For example, in mouse models, TRIM71 deficiency leads to neural tube closure defects and significantly suppresses the proliferation of neural progenitor cells (NPCs).7 Additionally, the SMARCC1 gene, which encodes a chromatin remodeling protein, plays a crucial role in the transcriptional regulation of NPC survival and telencephalic development. Research has shown that SMARCC1 knockout is strongly associated with the development of hydrocephalus and aqueductal stenosis.8 Further investigations indicate that TRIM71 mutations are primarily linked to communicating hydrocephalus, while SMARCC1 and PTCH1 mutations are more commonly associated with aqueductal stenosis.9 Therefore, patients with related genetic defects, whether born or acquired, are at greater risk of developing hydrocephalus.
Neuropathological injuries associated with hydrocephalus are related to mechanical and physiological factors which lead to white matter injury, decreased blood flow, and brain glial cell injury.10 In recent years, many studies have shown that the pathological changes of secondary hydrocephalus, especially after cerebral hemorrhage, infection, or trauma, are related to oxidative stress. Oxidative brain injury may be an important factor in the pathogenesis of hydrocephalus.11 Oxidative stress refers to a condition where the production of free radicals and reactive oxygen species (ROS) exceeds the capacity of the body's endogenous antioxidant defense and repair mechanisms to cope.12 Due to their high reactivity, free radicals and ROS can cause irreversible damage to lipids, proteins, and DNA within biological systems.11
The current treatment for hydrocephalus is primarily surgical, followed by supplemental medications or other therapy. Symptomatic hydrocephalus is usually treated by shunting in which cerebrospinal fluid is transferred to another body area.13 Although early cerebrospinal fluid shunts can restore injured periventricular white matter and regulate cerebral blood flow, axonal injury is irreversible.14, 15 In addition, bypass treatment may cause some common complications, especially obstruction and infection in infants, which may increase the mortality rate of the procedure.16, 17 Due to the inadequacy of existing treatments, new treatments are sought. Based on the oxidative stress in the development of hydrocephalus and the application of some antioxidants in the treatment of neurodegenerative diseases, the use of antioxidants may alleviate the pathological reaction of hydrocephalus. The purpose of this review is to review some antioxidants and potential drugs that can be used to treat hydrocephalus. We review the basic information about hydrocephalus and the pathogenesis of oxidative stress and its different signaling pathways. The effects of different antioxidants on hydrocephalus are also integrated and compared, particularly regarding future research on hydrocephalus treatment.
2 PATHOGENESIS
Hydrocephalus is a disease of the central nervous system in which cerebrospinal fluid accumulates excessively in the ventricles of the brain, often causing cognitive and physical impairments.18 The current widely accepted hypothesis of hydrocephalus is the circulation theory. According to the circulation theory,19 cerebrospinal fluid is produced mainly by the choroid plexus in the ventricle and flows from the lateral ventricle through the foramina Monro, the third ventricle, the aqueduct, and fourth ventricle, and then enters the subarachnoid space (SAS) through the foramina Luschka and the foramina Magendie, from which it is passively absorbed into the intracranial venous sinuses and into the bloodstream. Hydrocephalus results from an imbalance between the amounts of cerebrospinal fluid produced and absorbed. Any blockage of the normal flow of cerebrospinal fluid or its absorption can result in hydrocephalus.20 Hydrocephalus can be caused by any of three conditions: overproduction of CSF, obstacles to its absorption, or impairment of its circulatory pathways. Studies have shown that excessive production of cerebrospinal fluid is often caused by inflammation, where inflammatory factors are deposited on the choroid, causing its absorption to deteriorate.21 Secondary hydrocephalus may result from blocked CSF circulation due to intracranial tumors or trauma.22
Under normal circumstances, the synthesis and absorption of cerebrospinal fluid maintain a dynamic balance. Damage to brain tissue, for example, by infection or bleeding, leads to the production of excess oxides, causing an imbalance of the oxidative stress system, and a release of a large number of toxic substances into the CSF circulation.23 At the same time, excess oxide attacks the choroid plexus and arachnoid structures in the ventricle, damaging the normal circulation of cerebrospinal fluid. The cytotoxic effects of oxides also cause mitochondrial dysfunction and extracellular metal ion accumulation, and these changes may lead to excessive release of ROS.24 A further imbalance between ROS and the body's antioxidant defenses leads to the pathophysiology of hydrocephalus and may activate damaging inflammatory processes.
Recent research shows that the occurrence of congenital hydrocephalus is often related to transferrin. Transporters play a crucial role in maintaining the ionic balance between different regions of the brain. Among them, NKCC1, aquaporin-1 (AQP-1), and aquaporin-4 (AQP-4) are the three most closely associated with hydrocephalus and have been extensively studied.25 Research suggests that NKCC1 is the primary cause of excessive cerebrospinal fluid (CSF) secretion induced by inflammation. Loop diuretics, such as furosemide and bumetanide, which are sometimes used to control intracranial pressure (ICP), target this protein.26 It has been shown that in mice, NKCC1 can produce about half of the cerebrospinal fluid through water cotransport and targeted transport of ions in a process that is not affected by osmosis. This water cotransport is mainly realized through AQP-1 water channel protein, which is mainly distributed on the apical surface of choroid plexus epithelial cells (CPECs) but is not completely confined to it. Epithelial cells and CPECs play a crucial role in normal cerebrospinal fluid dynamics. The directional flow of cerebrospinal fluid is driven by the synchronized movement of ependymal cell cilia, and stable ependymal flow is essential for maintaining the patency of the cerebral aqueduct during development.27 Cilia are not only responsible for regulating the direction of cerebrospinal fluid (CSF) flow but also able to act as chemoreceptors to modulate the secretion of CSF. At least 20 genes are associated in people with syndromic hydrocephalus that encode proteins required for the normal function or biosynthesis of cilia and/or choroid plexus epithelium.28 However, while many genes have been associated with syndromic hydrocephalus, only a few are directly associated with congenital hydrocephalus (as a primary or sole feature). These genes include the L1 cell adhesion molecule (L1CAM), the sigma-2 subunit of adapter-associated protein complex 1 (AP1S2), the poly-PDZ structural domain protein (MPDZ), and a mutation in the discoidal helix structural domain-containing protein 88C (CCDC88C), which were identified in earlier studies.26
Studies have shown that hemorrhagic hydrocephalus and infected hydrocephalus may trigger an inflammatory response, which in turn disrupts normal ependymal and choroid plexus function. When foreign substances or microorganisms (e.g., viruses, bacteria, or fungi) are present in the cerebrospinal fluid, a nonspecific inflammatory response is usually induced. This response includes the release of cytokines such as IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α), as well as the production of local immunoglobulins by plasma cells.29 Animal studies have shown that injection of autologous blood into the lateral ventricle is sufficient to cause ventricular hypertrophy and activation of NF-kB pathways and cytokine production in choroid plexus epithelial cells, resulting in a more than threefold increase in cerebrospinal fluid secretion.30 It has been found that injection of autologous blood into the lateral ventricle triggers ventricular hypertrophy and activates NF-kB pathways in choroid plexus epithelial cells while promoting cytokine secretion, leading to a more than threefold increase in cerebrospinal fluid production.31 Both the inflammatory response and the accumulation of iron lead to free and active overproduction which further aggravates the pathological damage.
3 CAUSES AND PATHOLOGY OF OXIDATIVE STRESS
The pathways of oxidative stress are not clearly identified in the pathophysiology of hydrocephalus. However, several events leading to hydrocephalus, including infection, hemorrhage, and traumatic brain injury, have involved acute or chronic elevation of oxidative stress indicators.32-34 There are several mechanisms of oxidative stress.
3.1 Direct oxide action
The brain consumes about 20% of the oxygen in the whole body, although it accounts for only 2% of the total body mass, which means that the brain may produce more free radicals than other tissues.35 As a central regulator of cellular energy metabolism and redox (REDOX) homeostasis, damaged mitochondria become one of the major sources of intracellular reactive oxygen species (ROS).36 Mitochondrial dysfunction not only interferes with cellular metabolism but also leads to the overproduction of ROS, which further triggers an inflammatory response.36 When hydrocephalus occurs after bleeding, any depletion of reserve ATP results in the formation of several purine metabolites, such as xanthine and hypoxanthine.37 After reperfusion, these metabolites are decomposed by oxidation to form hydrogen peroxide and superoxide free radicals. At the same time, the changes in intracranial trace elements can also lead to the formation of peroxide. Experiments have shown that Cd concentrations are higher in the plasma and red blood cells of patients with hydrocephalus compared with those with brain tumors and that there is a strong correlation between the concentration of certain metals in patients' blood and biomarkers of oxidative stress38 (See Figure 1).

Sources of ROS in hydrocephalus. Hydrocephalus peroxide sources are divided into two main categories. One is caused by external factors, such as radiation damage to cells, and the other, accounting for a large proportion, is endogenous, including cellular NADPH oxidase, metabolic reactions in peroxisomes, ER stress, and unfolded protein response. The production of inflammatory bodies by pathological reactions, iron death, and mitochondrial damage produce large numbers of free radicals.
REDOX homeostasis is regulated by the antioxidant defense system, which plays a crucial role in eliminating various oxidants, including reactive oxygen species (ROS), lipid peroxides, and metals.39 In hydrocephalus following subarachnoid hemorrhage (SAH), excess reactive nitrogen species and ROS, including nitric oxide, superoxide anions, hydroxyl radicals, peroxynitrite, and hydrogen peroxide, have been found to attack non-enzymatic and enzymatic antioxidant defense systems.40 ROS production is thought to be a by-product of oxidative phosphorylation of mitochondria, which are the main targets of ROS-induced damage. Therefore, excessive ROS disrupt REDOX homeostasis. In addition, excessive oxidative stress can activate the autophagy system of cells, induce cell apoptosis, and cause further damage and destruction of brain cells.
Studies have shown that the most commonly recognized “secondary damage” in the study of traumatic brain injury mechanisms is the early post-traumatic increase in ROS, which leads to oxygen-induced damage to brain cell lipids and proteins.41 Sirtuins are a family of highly conserved NAD-dependent enzymes that play a key role in regulating mitochondrial functions in neuronal tissues.42 Sirtuin 3 (SIRT3) is mainly located in mitochondria where it can play a protective role by scavenging oxygen free radicals under pathophysiological conditions such as oxidative stress and metabolic disorders. Experimental studies have shown that SIRT3 expression is involved in the regulation of altered mitochondrial function, mitochondrial fusion and fission, and deacetylation of antioxidant enzymes such as superoxide dismutase 2 (SOD2),42 having a neuroprotective effect and promoting cell resistance to apoptosis. Mitochondrial-targeted antioxidants may have the potential to alleviate oxidative stress.39
3.2 Inflammatory response
When excessive oxidation occurs in intracerebroventricular cells, oxidative stress induces inflammation, which in turn can promote the further generation of oxidative stress; cellular damage leads to excessive production of free radicals and lipid peroxidation, irreversible damage to cellular physiological functions, and apoptosis, thus inducing functional brain damage.40 Brain injuries caused by various intracranial lesions are fundamentally stress responses, with stressors including increased hemodynamic load, elevated oxygen consumption, and changes in circulating levels of neurohormones, cytokines, and lipid factors. One key aspect of the stress response is the production of reactive oxygen species (ROS), which depletes the cell's antioxidant capacity by oxidizing glutathione and alters the intracellular redox (REDOX) state. This change in the REDOX state, driven by the activity of histone deacetylases (HDAC), modifies chromatin structure and initiates the expression of hypertrophic43 and pro-fibrotic44 gene programs, leading to transcriptional changes in neural cells. Additionally, the inflammation and oxidative stress triggered by these stimuli further activate matrix metalloproteinases (MMPs), which are involved in remodeling the extracellular matrix, and transitioning microglial cells from a resting to an active state.
Microglia also produce ROS, cytokines, and chemokines,45 functioning as key mediators of inflammation. Inflammatory responses may arise from pathogen exposure or environmental stressors such as ischemia and hemodynamic overload.44 These responses include adaptive immunity, marked by an increased presence of activated white blood cells, and innate immunity, critical for tissue repair, which elevates levels of inflammatory cytokines (e.g., TNF-α, IL-1, IL-6) and acute-phase proteins (e.g., C-reactive protein, serum amyloid A). Signals from inflammasome activation facilitate the recruitment of neutrophils and monocytes to damaged tissue. Once activated, inflammatory cells combat pathogens like bacteria and viruses by releasing ROS in the form of superoxide anions (O2−) and hydrogen peroxide (H2O2).46 These reactive substances are consumed in large quantities and more reactive oxygen species are produced.
Early brain injury (EBI) is an acute injury to the whole brain within 72 h after SAH and is the leading cause of death in SAH patients. Although the specific mechanism of EBI is not clearly defined, many findings suggest an important role of the inflammatory response in the development of EBI after SAH. The key to inflammation is the release of pro-inflammatory cytokines, such as IL-1β and TNF-α, and adhesion molecules.42 The pro-inflammatory cytokines released after SAH cause direct damage to peripheral neurons and may also enhance vascular permeability, thereby destroying the blood-brain barrier (BBB), increasing brain edema, and inducing apoptosis of neuronal cells to further exacerbate brain injury.47 Leukocyte migration requires adhesion molecules (e.g., ICAM-1), and the release of adhesion molecules can further aggravate brain inflammation, creating a vicious cycle.48 The inflammatory response is promoted by positive feedback with oxidative stress, which increases the apoptosis of intracerebroventricular cells and brain function damage, thus triggering a serious pathological chain reaction.49 In addition to the use of antioxidants to bring the REDOX system into balance as much as possible, anti-inflammatory factors can be used to regulate and decrease inflammation. Table 1 compiles some of the pathways involved in inflammatory responses to oxidative stress.
| Signal pathways | Model | State | Molecular mechanisms and outcomes | |
|---|---|---|---|---|
| In-vitro | In-vivo | |||
| TLR4/NF-κB | LPS-induced BV2 cells | — | Activation | ↑: TLR4, NF-κB, iNOS, IL-1β, IL-18 |
| JAK/STAT | — | SAH induced PHH rats | Activation | ↑: J AK2, STAT3, IL-6, IL-1β, TNF-α |
| MAPK/ULK-1 | — | TBI induced PHH rats | Activation | ↑: MAKP, TLR4 |
| ↓: ULK1, Beclin-1, LC3II/LC3I | ||||
| MAPK/mTOR | — | SAH induced PHH rats | Activation | ↑: MAPK, mTOR, TNF-α, IL-1β |
| ↓: Beclin-1, LC3II/LC3I, | ||||
| Nrf2/HO-1 | LPS-induced BV2 cells | — | Inhibition | ↑: NF-κB/p65, ROS, iNOS, NO, COX2, IL-6, TNF-α: ↓:Nrf2, HO-1 |
| PPARγ/SIRT3 | Fe-induced microvascular endothelial cells | — | Inhibition | ↓: PPARγ, SIRT3, FoxO3 |
- Abbreviations: BV2 cells, a kind of Murine microglia; LPS, lipopolysaccharide; PHH, Hydrocephalus; SAH, Subarachnoid hemorrhage; TBI, traumatic brain injur.
Current studies have reported that the inflammatory response is related to the expression of aquaporin-4 (AQP4) water channel in glial cells in the brain.50 When excessive inflammatory response exists in hydrocephalus in children, premature expression of AQP4 regulation leads to glial proliferation in astrocyte culture from hydrocephalic children.51 Differentiation of glial stem cells into astrocytes impinges on their proper function as scaffolds to guide cells into white matter, thereby disrupting the normal pathways of cerebrospinal fluid circulation.47
NF-κB, a transcription factor involved in regulating inflammation, is broadly present throughout the central nervous system. Studies have shown that NF-κB contributes to secondary brain injury following SAH by promoting the expression of pro-inflammatory cytokines and adhesion molecules.52 A growing body of research25 suggests that the upregulation of NF-κB, pro-inflammatory cytokines, and adhesion molecules in clinical and experimental SAH and inhibition of acute surges in NF-κB and inflammatory mediators can improve neurological recovery.
3.3 Mitochondrial dysfunction
Mitochondria are dynamic organelles responsible for ATP production and the regulation of cellular metabolic balance, survival, and death. They utilize oxygen to generate energy essential for sustaining normal cellular function.53 Mitochondrial ROS can impair the mitochondrial membrane potential leading to further ROS generation within the organelle. Elevated ROS levels in a limited number of mitochondria can propagate oxidative damage to others, eventually impacting the entire cell.50
In acquired hydrocephalus, mitochondrial dysfunction can lead to energy disturbance and metabolic abnormalities of cells, resulting in obstruction of CSF absorption and abnormal CSF circulation.51 Studies of idiopathic hydrocephalus, especially the dementia type, have shown a high prevalence of pathological mitochondria in the neuronal body and the presynaptic and synaptic endings, with increased mitochondrial aggregation and changes in the number of mitochondria-ER contact sites. Non-fused autophagy vacuoles are more abundant in the neuronal cell bodies of such patients, suggesting cell clearance failure. In addition, the size of postsynaptic density is reduced, which may be related to the decreased synaptic activity. Such pathological mitochondria also show an increase in their number, area, and area fraction during the astrocyte terminal stage.53, 54
Nicotinamide adenine dinucleotide (NAD+), also referred to as coenzyme I, is a critical signaling molecule that regulates intermediary metabolism.55 NAD + functions as a coenzyme in REDOX reactions and acts as a key substrate for sirtuins, which play a role in preserving mitochondrial homeostasis. NAD + supplementation enhances mitochondrial function, reduces ROS production, and limits the accumulation of damaged mitochondria. Studies have demonstrated that the NAD + precursor nicotinamide riboside can mitigate neuroinflammation in Alzheimer's disease mouse models.56 However, its potential to alleviate oxidative stress associated with hydrocephalus remains uncertain.57
3.4 Ischemic injury
Brain injury could play a significant role in the development of hydrocephalus. Hydrocephalus after TBI is often accompanied by severe pathological peroxidation reactions. It has been found that the number of microglia increases after cerebral hypoperfusion,58, 59 which may be related to mitochondrial dysfunction and release of ROS induced by cell ischemia.60, 61 Activated microglia secrete TNF-α, IL-1β, IL-6, and other pro-inflammatory factors, contributing to secondary brain injury. These factors penetrate the white matter, leading to neuronal damage and loss.62 When cerebral ischemia occurs, a more serious injury, in addition to hypoxia damage, is reperfusion injury. Recent studies recognize that iron contributes to pathological cell death during acute brain injury such as cerebral ischemia. Previous studies have shown that focal cerebral ischemia rapidly leads to an increase in free iron levels in ischemic tissue within an hour, and that iron, as an oxidizing agent, can lead to an imbalance of peroxides and antioxidants within the cell, thus acting as a trigger point to further induce a cascade of oxidative reactions.63 It has been proposed that certain exogenous iron death inhibitors could potentially reverse cerebral ischemia/reperfusion injury. After TBI, the formation of ROS and free radicals in brain tissue accelerates, promoting molecular damage processes (such as lipid peroxidation, DNA damage, and protein oxidation) and aggravating glutamate excitotoxicity, mitochondrial dysfunction, ion imbalance, and activation of cellular proteases.64-66 In addition, the force and kinetic energy released from brain tissue during traumatic impact also induce an immediate release of glutamate from neurons, altering the BBB permeability and leading to frequent hemorrhage and reduced cerebral blood flow in later stages. Excitotoxicity due to sustained glutamate release substantially alter ion homeostasis, leading to an increase in mitochondrial Ca2+.60 Calcium overload negatively affects mitochondrial function by binding the electron transport chain to oxidative phosphorylation to produce excess energy (as ATP), which leads to energy imbalance61 and contributes to ROS production.67 In addition, lipid peroxidation leads to free radicals' “stealing” electrons from cell membrane lipids, damaging cell membranes, and increasing the rate of apoptosis.68
The pathological reaction after SAH may also further induce hydrocephalus, thrombus formation may obstruct cerebrospinal fluid reflux, and the progression of subarachnoid fibrosis may aggravate the obstruction of cerebrospinal fluid.69 Blood products and transforming growth factors have long been recognized as key contributors to the pathophysiological processes following subarachnoid hemorrhage (SAH),70 including the development of chronic hydrocephalus. Strahle et al. observed cell death in a rat model through pathological analysis after intracerebroventricular injection of iron (ferrous chloride or ferric chloride) or lysed red blood cells.70, 71 Other studies have shown that iron oxide induces brain cell necrosis and BBB destruction.72 These and other clinical studies led to the original idea of “iron death”.73 Reducing the pathological response caused by excessive oxidation is expected to be a possible treatment for hydrocephalus.
Figure 2 summarizes and maps the mechanism of oxidative stress in the pathological process of hydrocephalus.
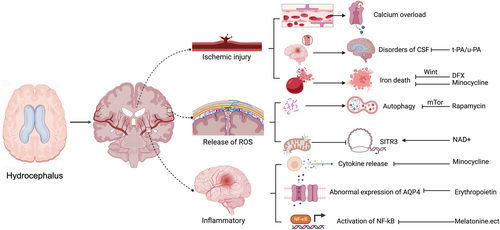
Role of oxidative stress in hydrocephalus pathogenesis. A large number of studies have shown that there is a relationship between oxidative stress and brain injury caused by hydrocephalus. Oxidative stress injury has three primary aspects: (1) Direct hemorrhagic injury. Blood clots form in cerebral vessels, the permeability of the blood-brain barrier is reduced, and the CSF seepage and reflux disorders hinder the return of CSF. Calcium overload and iron death caused by ischemia further aggravate the oxidative stress of hydrocephalus, forming a vicious cycle. DFX and minocycline can inhibit the process of brain death. (2) Direct destruction by oxides. ROS can lead to the destruction of intracellular proteins and induce autophagy which can be inhibited by rapamycin. ROS damages antioxidant enzymes in mitochondria and inhibits the expression of genes such as SIRT3, thus weakening the antioxidant and anti-apoptotic ability of cells. Supplemental NAD+ can promote the expression of this gene. (3) Inflammatory response induced by oxidative stress. Inflammation can directly promote the release of cytokines (TNF, IL-1, etc.) and the expression of NF-κB in the nucleus, further aggravating the inflammatory response. Minocycline can remove excessive cytokines, and melatonin and other antioxidants can activate the Nrf2 pathway to play a neuroprotective role. The increased ROS can also interact with inflammatory cells to interfere with the expression of AQP4 in neurons, and erythropoietin can induce its normal production.
4 AVENUES TO POTENTIAL ANTIOXIDANT THERAPIES
Research has shown that the pathological changes in hydrocephalus do not directly damage the antioxidant system but rather that excessive ROS lead to system overload, further reduction of antioxidants, and continuous production of peroxides. The search for methods to clear peroxides rather than repair antioxidant systems74 leads to the following pathways that can resist oxidative stress. Based on the occurrence of oxidative stress in hydrocephalus, these may have positive significance in alleviating the pathology of hydrocephalus.
4.1 Free radical scavengers
Studies have shown that the use of free radical scavengers can alleviate the pathological process of hydrocephalus. Here are some examples.
Germinal stromal intraventricular hemorrhage (GMH-IVH) is a common complication of hemorrhage in premature infants. After hemorrhage, blood is deposited in the interventricular space, erythrocytes are lysed, hemoglobin is released into the cerebrospinal fluid, and the detached hemoglobin undergoes redox reactions in which ferrous ions become ferric ions,75, 76 ultimately leading to increased release of heme.77 Free heme and iron ions are highly reactive and can cause DNA damage by inducing oxidative modifications in lipids, proteins, and cross-linked fragments.78 Heme, being lipophilic, can integrate into cell membranes, resulting in cell lysis.79
Direct scavenging of free radicals may slow the pathological process. Previous research80 has demonstrated that α1-microglobulin (A1M), a powerful histoprotective protein, functions through mechanisms such as heme binding, reductase activity, free radical scavenging, and mitochondrial binding,81, 82 thereby scavenging heme and free radicals. A1M uptake by mitochondria during the early stages of cell death inhibits heme and ROS-induced mitochondrial swelling.83 Recombinant human A1M exhibits similar functional properties to endogenous A1M found in human plasma (hA1M).80
The drug MCI-186, also known as edaravone, inhibits lipid peroxidation and eliminates free radicals. Numerous studies have highlighted its effectiveness in mitigating neuronal damage caused by conditions such as stroke, neonatal encephalopathy, acute cerebral hemorrhage, subarachnoid hemorrhage, amyotrophic lateral sclerosis, spinal cord injury, and epilepsy84; However, its application or potential role in treating hydrocephalus remains unreported.
As elucidated by Tan et al.,85 melatonin, a powerful free radical scavenger, has antioxidant capacity (up to 10 ROS per molecule) superior to that of many other antioxidants such as glutathione, vitamins E and C, and NADH. This effectiveness results from its cascade reaction in which its metabolites retain the ability to neutralize many toxic oxygen species. This efficacy against oxidative stress prevails in vivo as well as in vitro. Moreover, melatonin modulates oxidative stress and protects cells more effectively in vivo than under other antioxidants.86 Therefore, a melatonin molecule can detoxify multiple toxic ROS or NOS.
4.2 Regulation by peroxidase activity
A comparison of the regulation of multiple antioxidants in oxidative stress, targeting substances with therapeutic potential in neurological disorders, indicates that many antioxidants can slow pathological peroxidation by stimulating peroxidase and increasing its activity.87 Kinsman et al. (1997) highlighted the harmful impact of free radical nitric oxide (NO) on cell membranes.88 I Nitric oxide synthase (NOS), which regulates NO production, is known to influence cerebrospinal fluid and brain blood flow, with changes in NOS isoform expression observed during hydrocephalus progression.89 Research has also demonstrated that glutathione (GSH) plays a critical role in buffering reactive oxygen species (ROS) in brain tissue, while enzymes such as glutathione peroxidase (GSH-Px) and glutathione reductase (GSH-Rd) facilitate the elimination of hydrogen peroxide and organic peroxides.90 Melatonin not only directly scavenges reactive oxygen species (ROS) as a free radical scavenger but also reduces nitric oxide (NO) production by inhibiting nitric oxide synthase (NOS) activity. Additionally, melatonin enhances the activity of endogenous antioxidant enzymes.91
4.3 Activation of neuroprotective pathways
Some antioxidants can perform multifaceted, multilevel coupled regulation by modulating a particular antioxidant pathway. The activation of the Akt/Nrf2 signaling pathway enhances the expression of HO-1 and Nqo1, effectively mitigating oxidative stress damage in cells.92 Recent study93 has demonstrated a strong connection between the Akt/Nrf2 pathway and the regulation of oxidative stress within the nervous system. Edaravone administration within 2 days after FeCl3 intracerebroventricular injection was effective in activating the Akt/Nrf2 signaling pathway in the rat brain.93 Brain damage in these rats was assessed using brain water content, magnetic resonance imaging, neurological scoring, oxidative stress assay, protein blot analysis, and electron microscopy. These results suggest that edaravone treatment attenuates FeCl3-induced cerebral edema, ventricular dilation, and neurological injury.
As studied in cell models and rat models, the possible mechanism of the Akt/Nrf2 signaling pathway is prevention of oxidative stress by protecting septa cilia and neurons from oxidative damage, inhibiting ROS production, and upregulating SOD activity and protein expression of HO-1 and Nqo1. A number of drugs have the potential to improve oxidative stress and its pathological changes in hydrocephalus and possibly pave the way for future research (Table 2).80, 84, 89, 94-97
| Antioxidant | Mechanism of action | Primary target | Action effect | Applicable circumstances |
|---|---|---|---|---|
| Edaravone | Activation of Nrf2/HO-1 pathway | Keap-1 | Decrease water and SOD | Hydrocephalus following TBI, SAH |
| NRF-2 | ||||
| Melatonin | Activation of SIRT3 gene | Sirt3 caspase-3 | Increased BBB permeability; inhibition of NOS activity | |
| Astaxanthin | Regulation of Akt phosphorylation | Nrf2/HO-1 | Decrease in apoptosis; inhibition of inflammation | |
| Deferoxamine | Inhibition of Wnt1/Wnt3a | Wnt1/Wnt3am | Decreased iron and ferritin levels | Prevention of hydrocephalus after IVH |
| A1M(α1 microglobulin) | Mitochondrial protection | Hemoglobin/ROS | Reduces mitochondrial and inflammatory damage | Prevention of congenital hydrocephalus |
| Bevacizumab (anti VEGF) | Decreased expression of VEGF | VEGF | Reduce ependymal cell stripping, ciliary loss and ventricle enlargement | PTH |
5 DISCUSSION
Oxidative stress is a key factor in the development and progression of hydrocephalus. When intracranial trauma, intraventricular hemorrhage, or congenital injury occurs, the system of oxides and antioxidants in the brain becomes unbalanced, excessive oxygen free radicals directly attack the cytoplasm and nucleus, and autophagy is induced, resulting in more serious secondary damage. In addition to the direct attack of oxides, the induced inflammatory response in the later stage further promotes the pathological process of hydrocephalus, changing the permeability of the blood-brain barrier, and causing further derangement of cerebrospinal fluid circulation. Thrombosis caused by direct bleeding injury and massive destruction of red blood cells also plays an important role. Changes in the composition of cerebrospinal fluid and the released hemoglobin lead to iron overload in the cerebrospinal fluid and ferroptosis.
Antioxidant methods may have some positive significance for the treatment of hydrocephalus, but more studies are needed. Only in animal models has the application of some antioxidants been found to reduce the pathological degree of hydrocephalus to some extent. No studies have been conducted to determine whether antioxidant treatment is safer or more cost-effective than conventional treatment, but it is clear that antioxidant treatment has been shown to be effective in some animal studies.
Several new experimental drugs have been used to improve the postoperative symptoms of patients.98 Minocycline, an antibiotic with anti-inflammatory properties, was found to decrease the extent of ventricular dilatation in an experimental group of hydrocephalic animals compared with a control group. Minocycline treatment has been shown to effectively reduce gliosis and delay the progression of hydrocephalus, making it a promising adjunctive therapy for this condition.99 Melatonin is also a possible therapeutic agent. Studies using an artificially constructed model of hydrocephalus in mice investigated the effects of melatonin treatment on the production of ROS during pathogenesis. The results showed that the SAH + melatonin-treated group showed a lower increase in neurological scores and brain edema after SAH compared with the control group and a decrease in neuronal apoptosis. Molecular level results in the SAH + melatonin-treated group showed increased levels of SOD2, BAX, and caspase-3 cleavage and decreased levels of SIRT3 and Bcl-2 after SAH.100
Many basic studies have shown that single use of antioxidants has a positive effect, but whether the combination of antioxidants has a synergistic effect is controversial and more research is needed. In conclusion, Some evidence suggests that oxidative stress may serve as a potential therapeutic target for the treatment of hydrocephalus.
Enzymes that change during the development of hydrocephalus include thrombin, which plays a role in the early stages of brain injury following hemorrhage and contributes to the formation of hydrocephalus after intraventricular hemorrhage (IVH).101, 102 In a rat model, significant ventricular damage and severe ependymal ciliary damage were observed following intraventricular thrombin infusion.103 SOD and GSH decreased sharply, harming the antioxidant system.91
Some markers could strengthen the argument for antioxidant therapy. Numerous animal studies have shown that the KEAP-1/NRF-2/HO-1 pathway is activated in patients with hydrocephalus, leading to increased levels of NRF-2, HO-1, TAU, MPO, TOS, and OSI. A controlled experiment investigating the effects of oxidative stress and neuroinflammation in a middle cerebral artery occlusion (MCAO) model using male Sprague–Dawley rats examined the cholesterol-blocking drug ezetimibe (Eze) and its impact on downstream signal transduction. The following treatments were applied: the AMPK inhibitor dorsomorphin and Nrf2 siRNA. Administration of ezetimibe (Eze) via an intranasal route 1 hour after MCAO, led to an increase in p-AMPK expression and a reduction in cerebral infarction, neurological deficits, neutrophil infiltration, microglial/macrophage activation, DHE-positive cell counts, and malondialdehyde (MDA) levels.104 Specifically, Eze treatment resulted in elevated levels of p-AMPK, Nrf2, and HO-1. Meanwhile, the expression of Romo-1, thioredoxin-interacting protein (TXNIP), NOD-like receptor protein 3 (NLRP3), cleaved caspase-1, and IL-1β was significantly reduced. Identifying these biomarkers can evaluate the usefulness of the antioxidant, but more studies are needed.105
Few clinical studies have examined the treatment of hydrocephalus with antioxidants, and no conclusions on the long-term results and safety of this treatment have been obtained. However, animal studies have shown that compared with traditional medical treatment (acetazolamide, osmotic diuretics, etc.), antioxidants such as melatonin and edaravone reduce the area of lateral ventricle hydrops and the apoptosis of cerebellar tissue cells, as quantitated by the TUNEL method.93 The prognosis is better. Different antioxidants are administered differently according to the modeling method, but it can be confirmed that these drugs can cross the blood-brain barrier.106 The usual course of administration is 28 days.89 More clinical studies are needed to find suitable drugs and further explore the feasibility of this antioxidant therapy in hydrocephalus.
The application of antioxidants has played a significant role in the treatment of neurodegenerative diseases. Excessive ROS production is known to be linked to neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. Additionally, oxidative stress has the potential to modify the inflammatory response.107 Although oxidative stress and neuroinflammation are two completely different pathological events, they are interrelated and affect each other. Neuroprotective and reparative effects of antioxidant therapy for hydrocephalus have been shown in many animal experiments. This promises to become a new avenue for hydrocephalus treatment.
6 CONCLUSION
Hydrocephalus is a neurological disease that is difficult to cure and requires long-term continuous treatment. It often occurs in infants and the elderly. At the current research level, its pathogenesis is not fully understood, and there are great limitations in its treatment. Ischemia-hypoxia-induced oxidative stress may be one of the mechanisms of hydrocephalus injury, indicating a role for some antioxidants to mitigate its damaging mechanism. These conclusions may provide some implications for future research into hydrocephalus treatment via antioxidants.
The treatment of hydrocephalus has been studied for many years, but clinical drug treatment has not achieved great results. With an understanding of the multiple pathogenesis of hydrocephalus, researchers will have more treatment techniques and therapy options. Antioxidants may play an important role in the treatment of hydrocephalus, enabling progress in the treatment of hydrocephalus and in clinical research.
AUTHOR CONTRIBUTIONS
Jie Zhao: Conceptualization; formal analysis; investigation. Zhi Tang: Methodology; project administration; software, validation; visualization. Yuchu Jiang: Writing—original draft; writing—review and editing. Yijian Yang: Conceptualization; formal analysis; investigation. Junbo Liao: Formal analysis; investigation. Zhangjie Su: Methodology; project administration. Ahsan Muhammad Usman: Writing—original draft; writing—review and editing. Gelei Xiao: Project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Xiaoyu Chen: Supervision; validation; writing—review and editing.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 82171347), Hunan Provincial Natural Science Foundation of China (No. 2022JJ30971), the Scientific Research Project of Hunan Provincial Health Commission of China (No. 202204040024). The graphical abstract and Figure 1 were created with biorender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Date sharing is not applicable to this article as no new data were created or analyzed in this study.




