Streptococcus pneumoniae worsens cerebral ischemia via interleukin 1 and platelet glycoprotein Ibα
Abstract
Objective
Bacterial infection contributes to diverse noninfectious diseases and worsens outcome after stroke. Streptococcus pneumoniae, the most common infection in patients at risk of stroke, is a major cause of prolonged hospitalization and death of stroke patients, but how infection impacts clinical outcome is not known.
Methods
We induced sustained pulmonary infection by a human S. pneumoniae isolate in naive and comorbid rodents to investigate the effect of infection on vascular and inflammatory responses prior to and after cerebral ischemia.
Results
S. pneumoniae infection triggered atherogenesis, led to systemic induction of interleukin (IL) 1, and profoundly exacerbated (50–90%) ischemic brain injury in rats and mice, a response that was more severe in combination with old age and atherosclerosis. Systemic blockade of IL-1 with IL-1 receptor antagonist (IL-1Ra) fully reversed infection-induced exacerbation of brain injury and functional impairment caused by cerebral ischemia. We show that infection-induced systemic inflammation mediates its effects via increasing platelet activation and microvascular coagulation in the brain after cerebral ischemia, as confirmed by reduced brain injury in response to blockade of platelet glycoprotein (GP) Ibα. IL-1 and platelet-mediated signals converge on microglia, as both IL-1Ra and GPIbα blockade reversed the production of IL-1α by microglia in response to cerebral ischemia in infected animals.
Interpretation
S. pneumoniae infection augments atherosclerosis and exacerbates ischemic brain injury via IL-1 and platelet-mediated systemic inflammation. These mechanisms may contribute to diverse cardio- and cerebrovascular pathologies in humans. Ann Neurol 2014;75:670–683
Community-acquired pneumonia is a common condition that leads to prolonged hospitalization, impaired clinical outcome, and increased mortality of patients, especially those with compromised immunity, such as children, the elderly, and those with immune suppression as a result of disease or injury.1, 2 The gram-positive bacterium Streptococcus pneumoniae is a major human pathogen, and the most common cause of bacterial community-acquired pneumonia.3
Infection also contributes to the development of cardio- and cerebrovascular diseases and is associated with poor outcomes after heart disease and stroke.4-8 S. pneumoniae infection could contribute to stroke or cardioembolism by triggering the rupture of an atherosclerotic plaque in humans,7-10 and molecular signatures of common bacteria, including Streptococcus species, have been identified in atherosclerotic lesions.11, 12 Vaccination against S. pneumoniae reduces atherosclerosis; however, the effect of S. pneumoniae infection on atherogenesis has not been demonstrated experimentally.
Infection is a key risk factor for stroke in young patients,5 a cohort without any atherosclerotic burden, suggesting that infection could contribute to an ischemic event independently of inducing plaque rupture. Clinical data indicate that S. pneumoniae, together with other common infections such as Chlamydia pneumoniae and Haemophilus influenzae, also contributes to impaired outcome, prolonged hospitalization, and death after stroke.5 Experimental stroke in mice propagates pneumonia.13, 14 However, it is not known if sustained bacterial infections preceding an acute cerebrovascular event could trigger stroke or contribute to increased stroke pathophysiology. Therefore, the main hypothesis tested in the study was whether preceding infection by a clinically highly relevant bacterial strain, S. pneumoniae, induces systemic vascular inflammation and worsens stroke outcome and whether this occurs via inflammatory mechanisms that could be blocked therapeutically.
Here we demonstrate that S. pneumoniae infection promotes atherogenesis, and exacerbates inflammatory responses to cerebral ischemia, leading to increased brain injury via platelets and interleukin (IL) 1. Thus, we suggest that complications after acute vascular events is preceded by a rapid systemic inflammatory response and excessive coagulation induced by pre-existing infection that profoundly impairs outcome. This could be prevented by treatment with IL-1 receptor antagonist (IL-1Ra).
Materials and Methods
Animals
Experiments were carried out in adult male wild-type (WT; C57BL/6J; Harlan-Olac, Blackthorn, UK) and ApoE−/− (JAX 2052; Jackson Laboratory, Bar Harbor, ME) mice, aged 12 to 20 weeks. Male Wistar rats were 8 weeks old (250–300g). Aged (15 months) lean and (JCR:LA-cp; cp/cp) corpulent rats, which are obese, atherosclerotic, and insulin resistant,15 were also used. Animals were allowed free access to food and water and maintained under temperature-, humidity-, and light-controlled conditions. All animal procedures adhered to the UK Animals (Scientific Procedures) Act (1986), and experiments were performed in accordance with STAIR and ARRIVE guidelines.
Infection with S. pneumoniae
A protocol for doses and timing of S. pneumoniae (American Type Culture Collection [ATCC] 49619, capsular serotype 19F [Danish]) challenge was established to allow low-grade, indolent pulmonary exposure to S. pneumoniae infection that is sustained in animals for a period of 6 to 7 days. Animals were lightly anesthetized with 2.5% isoflurane, and S. pneumoniae or phosphate-buffered saline (PBS; mock infection, 50μl, pH 7.4) was slowly pipetted onto the nostrils to allow subsequent dissemination throughout the lungs. An ascending infectious challenge was used on day 0 (2 × 108cfu/ml), day 2 (4 × 108cfu/ml), and day 5 (8 × 108cfu/ml). Mild piloerection and slightly increased respiration rate were observed after infection in 30% of the animals, but none of the infected mice showed any neurological symptoms, including signs of meningitis, intracranial hemorrhage, or visible neuronal injury in the absence of experimental stroke. Animals showing signs of more severe infection, such as markedly reduced activity, weight loss, or a hunched appearance were excluded from further experiments (3 mice, 0 rats). Blood pH, pCO2, pO2, and O2 saturation were analyzed with a 248 Blood Gas Analyser (Siemens Healthcare Diagnostics, Surrey, UK).
Middle Cerebral Artery Occlusion
Middle cerebral artery occlusion (MCAo) was performed using the intraluminal filament technique as described earlier16 on mice infected with S. pneumoniae and age/weight-matched controls weighing 25 to 30g. In brief, animals were anesthetized with isoflurane and a silicone-coated monofilament (210μm tip diameter; Doccol, Redlands, CA) was introduced into the left external carotid artery and advanced along the internal carotid artery to occlude the MCA for 30 minutes. Occlusion was confirmed by a laser Doppler (Moor Instruments, Axminster, UK). During surgery, core temperature was maintained at 37 ± 0.5°C. Due to the large size and multiple comorbidities of corpulent rats, a distal MCAo model was used in all rat experiments to avoid extensive tissue dissection and mortality. Rats were anesthetized with isoflurane and subjected to cerebral ischemia by transient occlusion of the distal MCA for 60 minutes using a 10-0 suture as described earlier.17 After experimental stroke, 3 mice and 2 rats were excluded pre hoc due to improper occlusion of the MCA (n = 2) and surgical artifacts (n = 3).
Experimental Design
To investigate whether S. pneumoniae infection contributes to atherogenesis or vascular inflammation in different vascular beds, ApoE−/− and C57BL/6J mice aged 12 weeks were fed either an atherogenic Paigen diet (18.5% fat, 0.9% cholesterol, 0.5% cholate, 0.26% sodium; Special Diet Services, Witham, UK) or a normal chow diet (4.3% fat, 0.02% cholesterol; Special Diet Services) for 8 weeks prior to mock infection or infection with S. pneumoniae (8 experimental groups). Corpulent rats that spontaneously develop atherosclerosis as they age15 were fed a chow diet throughout. Mice and rats were sacrificed 6 or 7 days after the first infection, respectively.
For the experiments investigating how S. pneumoniae infection preceding experimental stroke influences outcome, all animals were fed a chow diet. Naive male C57BL/6J mice aged 14 to 16 weeks were infected with S. pneumoniae on day 0, day 2, and day 5 followed by 30 minutes of MCAo16 on day 6 and 4 hours or 24 hours of reperfusion. In separate experiments, mice were treated intraperitoneally with a single bolus of recombinant IL-1Ra (100mg/kg; Biovitrium, Stockholm, Sweden) or placebo (0.5% bovine serum albumin in PBS, pH 7.4). To block platelet adhesion through glycoprotein (GP) Ibα, mice received 100μg anti-GPIbα Fab (p0p/B),18 or control immunoglobulin (Ig) G intravenously. IL-1Ra or anti-GPIbα was administered 1 hour after 30-minute MCAo, and mice were euthanized at 24-hour reperfusion.
Aged lean and (JCR:LA-cp; cp/cp) corpulent rats or young Wistar rats were mock infected/infected with S. pneumoniae on day 0, day 2, and day 5 followed by 60 minutes of transient distal MCAo17 on day 7 and 24 hours of reperfusion. A group of Wistar rats received a single dose of IL-1Ra (100mg/kg) or placebo subcutaneously at 1 hour of reperfusion.
Tissue Processing
Under terminal anesthesia, blood was taken from the heart using 3.8% sodium citrate (1:10) as an anticoagulant. Animals were then perfused transcardially with saline followed by paraformaldehyde (PFA; 4% in PBS; Sigma-Aldrich, Dorset, UK). Brains were postfixed in 4% PFA at 4°C for 24 hours, and cryoprotected in sucrose/PBS. Coronal brain sections (20μm thick for mice and 30μm thick for rats) were cut on a sledge microtome (Bright series 8000; Bright Instruments, Huntingdon, UK). For cytokine measurement, saline-perfused brain, lung, spleen, and liver samples were homogenized, and processed as described earlier.19 Except for the lungs, S. Pneumoniae was not recovered from any other tissues examined, as expected in the case of the ATCC 49619 strain.
Measurement of Infarct Volume and Blood–Brain Barrier Damage
The volume of ischemic and blood–brain barrier (BBB) damage was measured as described previously and corrected for edema.16 Leakage of plasma-derived IgG (BBB damage) was detected with biotinylated horse antimouse IgG (1:500; Vector Laboratories, Burlingame, CA) followed by incubation with ABC solution (1:500; Vector), and the color was developed by diaminobenzidine tetrahydrochloride (DAB).
Analysis of Functional and Behavioral Outcome
Neurological status in mice was assessed according to a neurological grading score of increasing severity of deficit20: 0, no observable deficit; 1, torso flexion to right; 2, spontaneous circling to right; 3, leaning/falling to right; 4, no spontaneous movement. In rats an additive neurobehavioral scoring system was used as described earlier21: body position (completely flat, 0 to upright position, 4); spontaneous activity (none, 0 to repeated vigorous movement, 3); transfer arousal (coma, 0 to extremely excited, 5); gait (absolute incapacity, 0 to normal, 3); touch escape (none, 0 to extremely vigorous, 3), and positional passivity (no struggle when held, 0 to maximal struggle, 4).
Cytokine Measurement
Sample processing and protein determination were performed as described previously.19 In mice, plasma samples and saline-perfused brain, liver, spleen, and lung homogenates were measured for tumor necrosis factor (TNF) α, RANTES, chemokine (C-C motif) ligand 2, chemokine (C-X-C motif) ligand 1 (CXCL1), IL-6, IL-1β, IL-1α, IL-17α, interferon γ, granulocyte colony-stimulating factor (G-CSF), and IL-10 using CBA Flex Sets (BD Biosciences, Oxford, UK) according to the manufacturers protocol, with FACSArray and LRSII flow cytometers (BD Biosciences). Rat cytokines IL-1β, IL-6, CXCL1, and TNFα in plasma, liver, spleen, and lung were measured with DuoSet ELISA kits (R&D Systems, Abingdon, UK).
Flow Cytometry
Following Fc receptor blockade (antimouse CD16/CD32, clone 93; eBioscience, Hatfield, UK), cells were stained with cocktails of selected antibodies: T cells (CD4-FITC, CD8-PE, CD3-APC, CD45-PerCP-Cy5.5), B cells/dendritic cells (CD19-FITC, CD11c-PE, MHCII-APC ± CD45-PerCP-Cy5.5), B cells/granulocytes (CD19-FITC, Ly6G-PE, MHCII-APC, Ly6c-PerCP-Cy5.5), granulocytes/monocytes (CD11b-FITC, Ly6G-PE, Ly6c-PerCP-Cy5.5, CD115-APC), and platelets (CD61-FITC, CD42d-PE, CD62P-APC). Antibodies were purchased from eBioscience except for Ly6g-PE (1A8; BD Biosciences, Oxford, UK). Cells were acquired on an LSRII flow cytometer (BD Biosciences, Franklin Lake, NJ), and data were analyzed using FACS Diva software (BD Biosciences, Franklin Lake, NJ).
Immunohistochemistry and Immunofluorescence
Immunostaining was performed as described earlier.16 For immunohistochemistry, goat antimouse vascular cell adhesion molecule (VCAM) 1 (1:250; R&D Systems) and rabbit anti-Iba1 (1:1,000; Wako Chemicals, Neuss, Germany) primary antibodies were used, and the staining was developed with nickel DAB. For immunofluorescence, appropriate mixtures of rat antimouse CD45 (1:200; AbD Serotec, Oxford, UK), goat antimouse VCAM-1 (1:250; R&D Systems), rabbit anti-Iba1 (1:1,000; Wako Chemicals), goat anti–IL-1α (1:100; R&D Systems), or rat anti-CD41 (1:100; BD Biosciences, Oxford, UK) antibodies were used followed by the adequate fluorochrome-conjugated Alexa 594 or Alexa 488 donkey antisera (1:500; Invitrogen, Paisley, UK). Biotinylated tomato lectin (10μg/ml, Sigma-Aldrich) was visualized with streptavidin–Alexa 350 conjugate (Invitrogen). VCAM-positive blood vessels were counted in 3 random fields of view for each serial section (typically 8–10) in the cerebral cortex. Activated microglia were counted in the striatum, cerebral cortex, and hippocampus on 10 serial sections rostrocaudally (3–4 fields per section). CD45-positive cells were counted in the caudal lateral ventricle on 5 serial sections (starting at −1.58mm from Bregma). The ventricular ependyma was visualized by using VCAM immunofluorescence. Images were viewed on an Olympus (Tokyo, Japan) BX51 upright microscope using ×4, ×10, ×40, and ×60 objectives and captured using a Coolsnap ES camera (Photometrics, Tucson, AZ, USA) with MetaVue Software (Molecular Devices, Winnersh, UK). Images were processed and figures assembled in Adobe (San Jose, CA) Photoshop CS6. Except for mild changes to brightness and contrast applied uniformly across experimental groups, no other modifications to digital images has been made.
Staining of Aortae
Oil Red O staining was performed as described earlier.22 Plaque density was calculated on Oil Red O–stained en face preparations of the aorta by light microscopy/image capture analysis to determine the percentage lesion of the total area of the aorta. For E-selectin staining, antigen retrieval was performed followed by incubation with E-selectin antibody (1:25; Abcam, Cambridge, UK), secondary biotinylated antibody (1:200), and ABC / DAB for detection.
Quantitative Analysis and Statistics
Animals were randomized for the experiments by GraphPad Random Number Generator (http://graphpad.com/quickcalcs/randomN1.cfm). All quantitative analysis was performed under blinded conditions. Group sizes were determined by power calculation using DSS Research (https://www.dssresearch.com/) and Stattol.net (http://www.stattools.net/SSizAOV_Pgm.php) online resources, and based on results from previous studies using the MCAo model in mice or rats with identical age, gender, and genetic background (5% confidence level, 80% power, and an estimated 20–40% standard deviation, resulting in n = 5–8 for most experiments). Statistical power was also assessed in individual experiments with S. pneumoniae. Data were analyzed with Student t test (comparing 2 experimental groups, Prism 6.0; GraphPad, La Jolla, CA), and 1-way or 2-way analysis of variance (ANOVA), followed by Tukey and Bonferroni post hoc comparison, respectively (comparing ≥3 groups, Prism 6.0), or 3-way ANOVA followed by Scheffe post hoc test (comparing 8 groups, 3 variables; Fig 1D, F; analyzed with StatView; SAS Institute, Cary, NC). Neurological scores were analyzed with nonparametric t test (Mann–Whitney test, 2 groups), or Kruskal–Wallis test followed by Dunn multiple comparison (4 experimental groups). p < 0.05 was considered statistically significant.
Results
S. Pneumoniae Infection Induces Systemic Inflammation, Accelerates Atherosclerosis, and Results in Cerebrovascular Inflammation
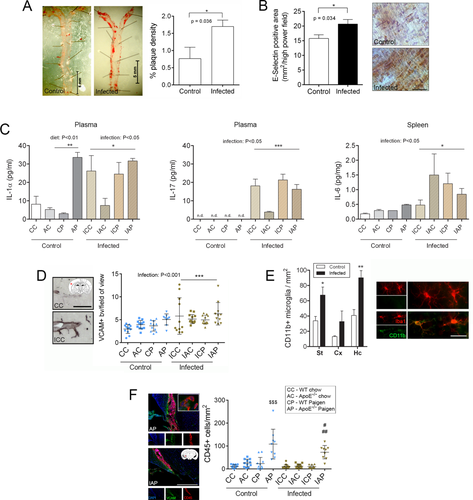
As atherosclerosis is the main risk factor for thromboembolism, and clinical data indicate an increased risk of thrombotic events in patients with pneumonia,7-10 we infected C57BL/6J and ApoE−/− mice fed a chow or a high-fat (Paigen) diet with increasing doses of a human isolate, S. pneumoniae ATCC 49619, to mimic sustained bacterial infection, and investigated cardio- and cerebrovascular responses. Infection caused a >2-fold increase in the number of aortic plaques within 6 days in high fat-fed WT mice, but had no additional effect on aortic plaque formation in ApoE−/− mice, which already showed advanced aortic plaques after 8 weeks of Paigen diet, as also observed in our earlier studies (see Fig 1).22, 23 The atherogenic effect of infection was also confirmed in aged, obese, and atherosclerotic corpulent rats based on increased E-selectin expression by the aortic endothelium. Analysis of peripheral cytokines showed elevated circulating IL-1α and IL-17 levels and increased IL-6 production in the spleen in response to infection in mice. Atherogenic diet also increased plasma IL-1α, but no additive effect of infection was observed.
Next, we investigated whether infection caused cerebrovascular inflammation. VCAM levels on the cerebrovascular endothelium increased significantly in response to infection, whereas atherogenic diet caused vascular activation only in ApoE−/− mice, as we reported earlier (see Fig 1).22, 23 There was no synergistic effect between diet and infection. Microglial CD11b levels were elevated in WT mice in response to infection, but the number of Iba1+ microglia was not altered. We reported earlier that atherogenic diet induces leukocyte accumulation in the choroid plexus that is most apparent in atherosclerosis-prone ApoE−/− mice.22 S. pneumoniae infection significantly reduced (by 40%) diet-induced accumulation of CD45+ cells in the lateral ventricle, indicating that infection-induced systemic responses modify inflammatory changes in the brain. No acute cardio- or cerebrovascular events were observed in mice or rats during the course of infection.
Preceding S. Pneumoniae Infection Exacerbates Brain Injury and Potentiates BBB Breakdown in Association with Age and Comorbidities
Sustained S. pneumoniae infection in young, healthy C57BL/6J mice prior to experimental stroke resulted in a significant (60%) exacerbation of brain injury induced by cerebral ischemia and markedly impaired neurological outcome 24 hours after reperfusion (Fig 2). Infected mice showed a trend for reduced O2 saturation and had significantly reduced pO2 levels in the blood before experimental stroke compared to uninfected mice, as expected in animals with pneumonia, similarly to patients with community- or hospital-acquired pneumonia.24
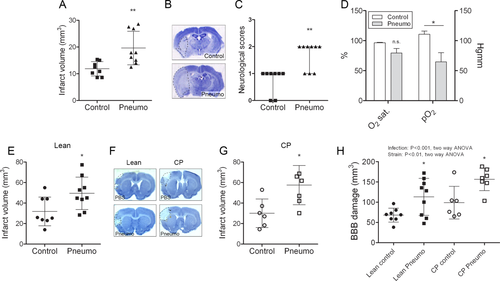
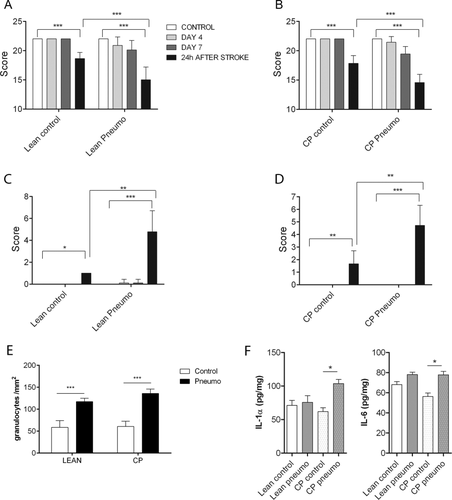
To investigate the effect of S. pneumoniae infection on stroke outcome in a comorbid rodent model, we infected aged lean and corpulent (obese and atherosclerotic) rats prior to MCAo. Infection significantly exacerbated brain injury in both lean (by 55%) and corpulent (by 90%) rats (see Fig 2). Aged, infected rats had markedly increased BBB injury as identified by parenchymal infiltration of plasma-derived IgG. Corpulent rats displayed significantly higher (by 40%) BBB breakdown in response to infection compared to lean rats. Infection worsened both behavioral outcome and sensory–motor functions after experimental stroke, which paralleled increased granulocyte recruitment in the ischemic hemisphere (Fig 3). Splenic IL-1α and IL-6 levels were increased after infection in corpulent, but not lean rats, indicating that animals with chronic vascular disease develop an exaggerated systemic inflammatory response to S. pneumoniae after cerebral ischemia.
S. Pneumoniae Infection Causes Granulocytosis and IL-1–Mediated Systemic Inflammatory Response
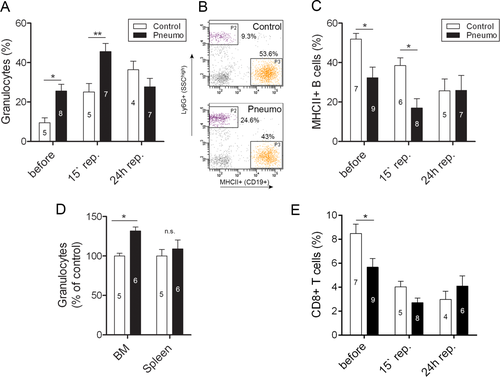
Infected mice had elevated circulating granulocytes prior to experimental stroke, which was associated with reduced B-cell numbers in the blood (Fig 4). Bone marrow granulocyte levels were also elevated, indicating that although the infection is localized to the lung, infection-induced systemic inflammatory responses involve increased granulopoiesis in the bone marrow and release of granulocytes into the circulation. In addition to innate immune mechanisms, T cells play a role in defense against S. pneumoniae infection.25-27 However, we found that infected mice had reduced CD8+ T cells in the blood prior to cerebral ischemia.
Next, we investigated cytokine changes in the blood and in peripheral organs after infection and cerebral ischemia. S. pneumoniae infection led to a 30- to 80-fold elevation in IL-1α and IL-1β levels in the lung, where the infection was localized, 4 hours after MCAo, paralleled by an increase in G-CSF, IL-6, and TNFα levels (20–30-fold, Fig 5A). RANTES and TNFα levels were increased in the liver in response to infection; IL-1α, IL-1β, G-CSF, peaked 4 hours after cerebral ischemia (see Fig 5B) in infected animals. No cytokine changes were detected in the spleen between control and infected mice (not shown).
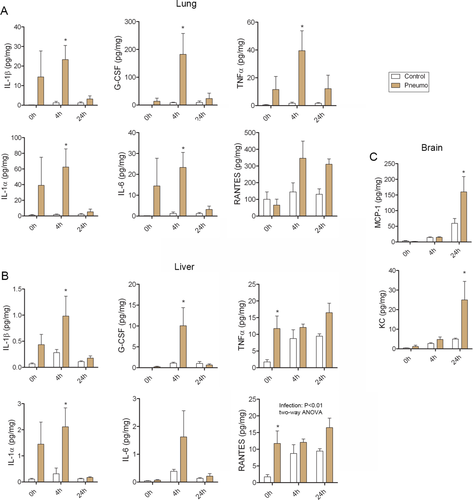
In infected mice, experimental stroke led to 2.5-fold higher levels of MCP-1 and 5-fold higher levels of KC in the brain compared to controls (see Fig 5C), two key chemokines responsible for the recruitment of monocytes and granulocytes to the brain.28, 29
S. Pneumoniae Infection-Induced Brain Injury is IL-1 Dependent
In mice, blocking IL-1 actions by IL-1Ra fully reversed the increase in ischemic brain injury induced by infection and restored impairment in sensory–motor performance (Fig 6). Infection induced a 3-fold increase in BBB injury, which was reduced by 40% after IL-1Ra treatment. We have reported previously that chronic infection preceding stroke increases granulocyte numbers in the spleen, and that granulocytes contribute to ischemic injury and BBB breakdown mediated by systemic inflammation.16, 30 IL-1Ra significantly reduced granulocyte numbers in the spleen (by >50%) and had similar effects on CD4+ T cells in the bone marrow, irrespective of infection status.
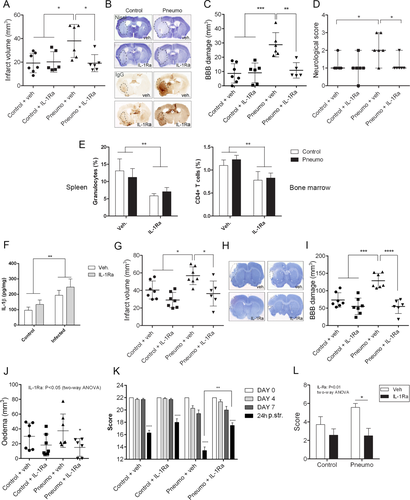
Similarly to mice, Wistar rats displayed significantly elevated IL-1β levels (2-fold) in the liver in response to infection, confirming the development of a systemic inflammatory response (see Fig 6). Infection significantly (by 40%) increased brain injury induced by cerebral ischemia. IL-1Ra administration fully reversed the infection-induced increase in brain injury and BBB breakdown after cerebral ischemia, suggesting that impaired outcome after stroke in response to S. pneumonia is IL-1 dependent in both rodent models. IL-1Ra treatment after cerebral ischemia also reversed brain edema in infected rats, improved behavioral outcome, and rescued motor performance.
S. Pneumoniae Infection Drives Brain Injury Via Platelet-Dependent Inflammatory Responses
As infection could facilitate coagulatory processes,5 we examined platelet aggregation in the brain. We found an increased number of platelet aggregates in the area of the infarct in infected mice after experimental stroke, and this was also confirmed in the ipsilateral hemisphere of both aged lean and corpulent rats in response to infection (Fig 7). Platelet accumulation was observed mostly in small cerebral arteries, resulting in partial or complete coverage of the vascular lumen, and was not affected by IL-1Ra. Flow cytometric analysis confirmed increased platelet activation in infected mice prior to MCAo as identified by higher levels of P-selectin (CD62P) on CD42d/CD61-positive circulating platelets. Blockade of platelet GPIbα (using a specific Fab fragment, which does not induce depletion of platelets18) significantly reduced infarct size and BBB injury, and improved neurological outcome in infected mice, but did not alter platelet accumulation in the brain, suggesting an inflammatory role for platelets. GPIbα blockade did not facilitate intracranial bleedings in infected mice. Only 2 mice receiving control IgG and 1 mouse receiving anti-GPIbα Fab showed minor hemorrhagic transformation (<0.1% of the volume of the infarct). GPIbα blockade did not mediate protection against the small, primarily striatal injury in uninfected mice.
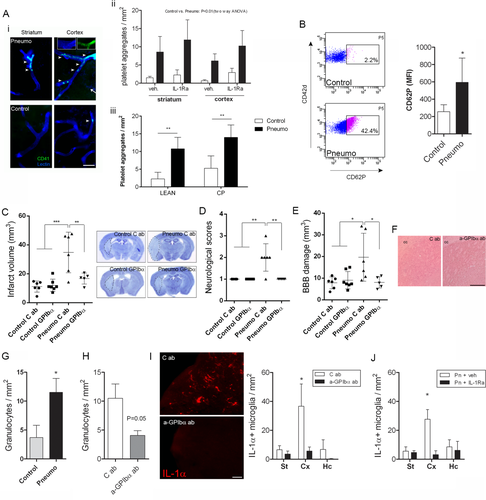
Blockade of platelet GPIbα reduced infection-induced granulocyte recruitment in the brain by 50% (see Fig 7). As both IL-1Ra and blockade of GPIbα reversed brain injury in infected mice, we further investigated whether systemic inflammatory changes alter microglial activation, which are the primary inflammatory cells in the brain. Microglial (lectin+/Iba1+) IL-1α was highly expressed in the ipsilateral hemisphere in infected mice after cerebral ischemia, which was significantly reduced after platelet GPIbα blockade. This reduction was most apparent in the ipsilateral cerebral cortex (see Fig 6H). Analysis of brain sections in infected mice also showed a comparable reduction in cortical IL-1α–positive microglia in response to IL-1Ra administration (see Fig 7J), indicating that IL-1– and platelet-dependent mechanisms contribute to microglial inflammatory changes and brain injury in response to infection after cerebral ischemia. We have also assessed whether infection could contribute to increased BBB injury, microglial IL-1α expression, or granulocyte recruitment independently of increased infarct size. Mice displaying the smallest infarcts from the infection group across different studies had comparable infarct size to those showing the largest infarct volumes in the control group (21.2 ± 6mm2 vs 22.8 ± 6.8mm2, respectively, n = 5). Granulocyte numbers were not (5 ± 1 cells/mm2 vs 5 ± 2 cells/mm2), but BBB injury volume (22.9 ± 3.8mm2 vs 13.8 ± 4.8mm2, p < 0.01) and number of IL-1α–positive microglia (18 ± 7 cells/mm2 vs 5 ± 4 cells/mm2, p < 0.01) were significantly higher in infected animals with even small infarcts compared to control mice after cerebral ischemia.
Discussion
We present evidence that sustained pulmonary S. pneumoniae infection, the most common cause of bacterial community-acquired pneumonia, generates an IL-1–mediated systemic inflammatory response involving granulocytosis and platelet activation that accelerates atherogenesis and leads to cerebrovascular inflammation. S. pneumoniae–induced systemic inflammation preceding an acute cerebrovascular event profoundly exacerbates inflammation in the brain via IL-1– and platelet-dependent mechanisms that augment microglial IL-1α production and lead to increased neuronal injury.
Clinical data indicate that infection contributes to the development of vascular disease, is a trigger for an acute vascular event, and/or contributes to outcome once an acute vascular event has occurred. In patients, infection could mediate all of these effects in both heart disease and stroke,5, 8, 10 although the mechanisms are not known, and current experimental evidence is insufficient. Chlamydia pneumoniae infection has been shown to induce an unstable atherosclerotic plaque phenotype in low-density lipoprotein receptor, ApoE double-knockout mice.31 There is clinical evidence to show that S. pneumoniae contributes to atherogenesis,11, 12 although the efficacy of antibiotic treatment on heart disease is controversial.32 However, no experimental studies have addressed the role of sustained S. pneumoniae infection in plaque growth or plaque rupture to date. We show that systemic effects of infection induce inflammation in distant vascular beds, and lead to vascular pathologies in both the aorta and the brain, although the extent of the response in individual mice was different. Our study was not designed to specifically investigate plaque rupture, hence it is not surprising that no acute cardio- or cerebrovascular events were observed in infected, atherosclerotic rodents. In contrast, our data clearly indicate the acceleration of atherogenesis by S. pneumoniae infection in atherosclerosis-prone rodent models, arguing for a contribution of S. pneumoniae to vascular disease.
To examine the effects of sustained and localized pulmonary S. pneumoniae infection on systemic inflammatory changes and stroke outcome, we infected mice intranasally with S. pneumoniae ATCC 49619 serotype 19F (Danish), a clinically relevant, human isolate. Because major virulence factors such as pneumolysin are conserved across all S. pneumoniae strains, we used mock infection in control mice to account for any unexpected effects caused by the inoculum in the lungs. In general, serotype 19F isolates are associated with colonization of mucosa,33 and are less commonly associated with invasive disease (serotype 19F is included in the 7-valent conjugated pneumococcal polysaccharide vaccine PCV-7). Lack of invasiveness of ATCC 49619 was also confirmed in our experimental model. The infectious challenge was titrated over 5 days to maximize the stimulation of the immune system while minimizing the incidence of invasive disease in the acute phase. Although in this model the infection is localized to the lung, we found marked systemic inflammatory changes in several organs, dominated by the key proinflammatory cytokine, IL-1. It has been shown that serious pulmonary infection can cause multiorgan dysfunction and a high level of mortality.34 We developed our inoculation protocol to result in sustained infection over a course of several days using intermittent sublethal challenges, without causing major weight loss, fever, behavioral alterations, or death.
In patients, infections preceding a stroke are associated with increased stroke risk and result in impaired outcome, similarly to infections that develop poststroke.5, 8, 35, 36 Our data clearly show that although pneumonia was associated with reduced pO2 levels (with O2 saturation unchanged) prior to stroke that could contribute to poor outcome, intervention against both IL-1 and platelet GPIbα reversed infection-induced cerebrovascular inflammation, neuronal injury, and impaired functional outcome, arguing for a major role of inflammation in brain pathologies caused by systemic effects of pulmonary S. pneumoniae infection. It is also possible that local and/or systemic inflammation could compromise perfusion and oxygen uptake/release in various organs including the lung or the brain. However, mild hypoxia due to lung inflammation was found to have no direct effect on infarct size.37 Nevertheless, our results strongly suggest that patients with stroke and pneumonia could benefit substantially from anti-inflammatory therapies, such as IL-1Ra. We investigated central and systemic inflammatory mechanisms in detail to explain how IL-1 can mediate injury after infection. As in our rodent model, infection-induced neutrophil and platelet activation has been documented in patients with pneumococcus-induced lung infection.26, 38 Granulocyte levels were increased after infection and cerebral ischemia in the brain, and were marginally reduced by GPIbα blockade, but not IL-1Ra, indicating that granulocyte responses might not fully explain the effect of infection on brain injury. Granulocyte numbers were not increased in infected mice independently of infarct size either. Although after IL-1–mediated cerebrovascular transmigration granulocytes acquire a neurotoxic phenotype, and IL-1 actions can worsen injury in the brain via granulocytes in vivo,30, 39 increased numbers of parenchymal granulocytes in the current experimental model might be indirectly associated with infection status and thus bigger infarcts. Nevertheless, systemic or perivascular granulocyte responses might mediate brain injury independently of the cells in the brain parenchyma, although this was not tested experimentally in the present study. In contrast, both platelet intervention and IL-1Ra uniformly reversed microglial IL-1α after cerebral ischemia in infected mice, suggesting that altered microglial activation (which was evident even prior to infection) could contribute to infection-induced inflammatory responses. Similarly, increased BBB injury was evident in infected mice with even smaller infarcts, supporting our earlier observations that systemic inflammatory mechanisms could mediate inflammation and injury in the brain independently of changes in infarct size.40
Vascular activation after infection, and increased platelet aggregation in infected mice after cerebral ischemia, suggested that inflammatory signals in the brain might be mediated by activated platelets. According to a recent study, S. pneumoniae can induce platelet aggregation in a strain-specific manner via toll-like receptor 2, which is dependent on GPIIb/IIIa, but is not affected by aspirin.41 In addition, our previous data indicated that a chronic infection resulting in a systemic, Th1-polarized immune response leads to the accumulation of platelets in brain microvessels after cerebral ischemia,16 although the functional role of platelets in the brain has not been investigated earlier after infection. GPIbα blockade did not reduce microvascular platelet aggregates in the ischemic brain after S. pneumoniae infection, arguing for an inflammatory role for platelet GPIbα-mediated interactions in augmenting cerebrovascular pathologies. We reported recently that platelet-derived IL-1α can induce cerebrovascular inflammation.42 Activated platelets release IL-1 and/or IL-1–containing microparticles upon interacting with the endothelium.42, 43
We have demonstrated previously that peripheral IL-1 administration exacerbates brain injury,30 and inflammatory mechanisms reportedly contribute to cerebral ischemia even in the absence of systemic inflammation.28, 29 Both GPIbα blockade and IL-1Ra have been shown to be protective in uninfected animals.17, 18 However, due to the small cerebral injury (that mostly corresponded to the core of the infarct) seen in uninfected animals, we did not expect these interventions to significantly reduce brain injury in control animals in the current experimental model. Importantly, our present results indicate that IL-1–dependent systemic inflammation and pathologies in the central nervous system can develop in response to sustained bacterial infection in vivo, and this disease mechanism is central to the pathologies caused by a clinically important gram-positive bacterium, S. pneumoniae. It is likely that such mechanisms could be important in other vascular, noncommunicable diseases as well, such as myocardial infarction, and liver or kidney ischemia.
In conclusion, our data identify IL-1 as a key mediator of infection-induced brain injury and indicate that selective targeting of IL-1– and platelet-mediated mechanisms could be therapeutically useful to prevent infection-induced thromboinflammatory mechanisms, which predispose to acute vascular events and lead to profound impairment in outcome after stroke.
Acknowledgment
Funding was provided by the Medical Research Council (NJR MRC Research Professorship; S.M.A.) and the European Union's Seventh Framework Programme (FP7/2008-2013) under grant agreements 201024 and 202213 (European Stroke Network; N.J.R., A.D.).
We thank OTKA K 109743 (A.D., B.M.), TÁMOP-4.2.4.A/2–11/1-2012-0001 (A.D.), and the Hungarian Brain Research Program KTIA 13 NAP-A-I/3 (A.D.) for their support; the Bioimaging facility of the University of Manchester; and J. Russell and S. Proctor for the corpulent rats.
Authorship
A.D., N.J.R., and S.M.A. designed research. A.D., J.M.P., C.D., A.S., P.W., K.N.M., B.R., J.C., H.C., and B.M. performed research. P.W., D.H.D., S.F., and B.N. contributed new reagents/analytic tools. A.D., J.M.P., C.D., A.S., P.W., K.N.M., B.R., J.C., H.C., and B.M. analyzed data. A.D., N.J.R., and S.M.A. wrote the article. A.D. and J.M.P. contributed equally.
Potential Conflicts of Interest
D.H.D.: funding, Wellcome Trust, MRC, GSK (no direct conflict of interest in the results of this research). N.J.R.: nonexecutive director of AstraZeneca (no involvement in this work).




