Glycopattern comparison of sperm and exosomes from conventional in vitro fertilization and rescue intracytoplasmic sperm injection: Enhancing sperm motility through exosome-mediated glycan modulation
Muhammad Anas Ramzan and Zhedong Yang contributed equally to this study.
Abstract
Infertility is a global issue, with male factors contributing to approximately 50% of cases. While assisted reproductive technologies (ART) resolve 30%–50% of cases, 2%–5% of IVF failures require rescue intracytoplasmic sperm injection (RICSI). Sperm glycocalyx is critical for capacitation and fertilization, and sperm-derived exosomes, essential for cell communication, remain underexplored. This study used lectin microarrays and fluorescence lectin histochemistry to analyze glycopatterns in sperm and exosomes from IVF and RICSI groups, and assessed RICSI sperm motility and glycan expression after exposure to IVF-derived exosomes. Clinical data from 2023 at Northwest Women and Children's Hospital, China, showed that RICSI couples had longer infertility durations, and younger ages, required lower gonadotropin doses, and retrieved more oocytes, despite lower sperm quality. However, pregnancy rates were similar between RICSI and IVF groups. Glycopattern analysis revealed that αGalNAc, Tn antigen, and GalNAcα1-3(Fucα1-2)Gal (DBA-recognized) glycans were downregulated in RICSI sperm, while Galβ1-3GalNAcα-Ser/Thr (T), GalNAcα-Ser/Thr (Tn), and sialyl-T (Jacalin-recognized) were upregulated in RICSI exosomes compared to IVF. Exosomes showed higher expression of PHA-L-, SBA-, and UEA-I-bound glycans, but lower AAL-bound glycans than sperm. DBA is primarily bound to the neck, acrosomal region, plasma membrane, and tail of IVF sperm, but its binding was significantly reduced in RICSI sperm. Functionally, IVF exosomes enhanced RICSI sperm motility in a dose-dependent manner by upregulating DBA-recognized glycans. These findings provide insights into biomarkers, IVF optimization, and glycosylation's role in sperm and exosome-related infertility, suggesting exosome-based therapy for improving RICSI sperm motility.
1 INTRODUCTION
Infertility, affects about 15% of the global population, one in six couples of reproductive age, with about 50% of cases attributed to male factors alone or in combination with female factors.1 In 85%–90% of cases, infertility can be effectively addressed through lifestyle modifications, medication, assisted reproductive technology (ART), or surgical interventions, enabling conception.2 Assisted reproductive technologies (ART) encompass medical procedures designed to assist individuals or couples in achieving pregnancy when natural conception is unattainable. The most common ART methods include intrauterine insemination (IUI) and in vitro fertilization (IVF). Conventional IVF, also known as first-generation IVF, involves the co-incubation of eggs and sperm in a controlled laboratory environment to facilitate natural fertilization. In contrast, intracytoplasmic sperm injection (ICSI), or second-generation IVF, entails the direct injection of a single sperm into an egg, primarily utilized in cases of severe male infertility. However, 2%–5% of conventional IVF cycles result in total or partial fertilization failure, necessitating rescue ICSI (RICSI). This highlights the potential influence of unidentified factors on fertilization success. Compared to direct ICSI, RICSI introduces additional procedural steps, increased emotional burden, and higher treatment costs following conventional IVF failure. Moreover, assessing IVF failures demands specialized expertise, and repeated manipulation of oocytes may compromise fertilization, embryo development, and long-term outcomes. To mitigate these challenges, identifying reliable sperm biomarkers and elucidating the underlying mechanisms are critical for optimizing IVF strategy selection and minimizing unnecessary resource utilization.3
Sperm, a renewable biological sample produced by males, carries essential genetic information, proteins, lipids, and carbohydrates. Numerous studies have demonstrated that sperm vitality and composition are crucial for successful fertilization. For instance, sperm DNA fragmentation has been linked to male infertility and reproductive failure.4 In mice, sperm proteins such as SOF1, TMEM95, and SPACA6 are required for sperm–oocyte fusion.5 Additionally, disturbances in the physicochemical properties of the sperm plasma membrane, potentially mediated by proteins like ezrin, Cdc42, and tetraspanin CD9, may contribute to idiopathic asthenozoospermia by affecting cytoskeletal reorganization.6 Spermatozoa are synthesized in the testes in an inactive state, with a thick glycocalyx, and pass through the epididymis for further modifications via glycosylation, deglycosylation, and integration to achieve maturation. Sperm capacitation and subsequent fertilization require the redistribution of glycoconjugates and significant modification of the sperm surface glycocalyx. Furthermore, glycoproteins and glycans in seminal plasma play a role in maintaining sperm structure and stability.7 Thus, any aberrant glycosylation (e.g., sialylation) during the sperm life cycle can contribute to male infertility, though our understanding of this process remains limited.8
Protein glycosylation is a critical post-translational modification that affects more than half of all known proteins. In eukaryotic cells, the most common types of glycosylation include N-glycosylation, O-glycosylation, and glycosylphosphatidylinositol (GPI)-anchored glycans, which are crucial for a variety of cellular processes such as protein folding, stability, transport, and protein−protein interactions. Aberrant glycosylation is closely associated with the onset and progression of numerous significant human diseases, including cancer, pathogen infections, inflammatory responses, cardiovascular diseases, and neuropsychiatric disorders.9 Furthermore, dysregulated protein glycosylation can contribute to adverse pregnancy outcomes, such as missed abortions and recurrent spontaneous abortions.10 In terms of reproductive biology, while complex N-glycans are essential for mammalian spermatogenesis, core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 appear to be dispensable.11 However, little is known about the specific glycoprotein patterns in sperm that fail in fertilization using conventional IVF but succeed with RICSI. Further investigation into these glycopatterns may offer valuable insights into the mechanisms underlying fertilization failure.
Exosomes are 30–150 nm microvesicles released by various cells into the extracellular space. They contain lipids, proteins, nucleic acids, and carbohydrates, and are capable of inducing physiological and pathological processes in target cells. In the reproductive system, exosomes are found in seminal plasma, follicular fluid, the endometrium, and epididymal fluid, where they influence processes such as the acrosomal reaction, embryo implantation, sperm capacitation, and gametogenesis.12 They carry specific molecules that regulate sperm and egg function, thereby affecting fertility. Human seminal exosomes contain many essential proteins. Gene ontology (GO) analysis from previous research has shown that most of the proteins in exosomes are involved in transport, cell development, and energy pathways.13 Unlike seminal exosomes, sperm-derived exosomes are released specifically by sperm cells. They more accurately reflect the composition and function of sperm, yet have been rarely studied.
Like cells, exosome surfaces are densely covered with glycoconjugates (proteoglycans and glycoproteins), and several tumor-associated glycan alterations have been identified in cancer exosomes.14 In this study, we precisely profiled the glycopatterns of proteins from sperm and sperm-derived exosomes in patients undergoing conventional IVF and RICSI. We identified distinct glycan alterations and distributions between the IVF and RICSI groups. Moreover, IVF-derived exosomes significantly improved key motility parameters of RICSI sperm in a dose-dependent manner by upregulating DBA-recognized glycans on their surface. These findings provide valuable insights into potential biomarkers and the glycosylation mechanisms underlying infertility.
2 METHODS
2.1 Study approval and subject grouping
The collection and use of all human semen specimens for this research were approved by the Ethical Committee of Northwest Women and Children's Hospital (Approval # 2024–128), affiliated with Xi'an Jiaotong University (Xi'an, China). Written informed consent was obtained from all patients for the collection of their semen. Fifty patients undergoing conventional IVF (IVF group) and 50 patients receiving rescued ICSI (RICSI group) were recruited from the Northwest Women and Children's Hospital, affiliated with Xi'an Jiaotong University. These patients, aged between 25 and 45, were diagnosed by reproductive specialists as needing IVF treatment. The detailed grouping method is described below, and the clinical information of the enrolled couples is shown in Table 1.
| Variables | Successful fertilization (IVF) | Fertilization failure (proceed for RICSI) | *p-value |
|---|---|---|---|
| Number of cycles | 8678 | 183 | — |
| Number of cycles per couple | 1.28 ± 0.74 | 1.05 ± 0.23 | .000 |
| Years of infertility (years) | 3.55 ± 2.67 | 4.12 ± 2.50 | .004 |
| Female age (years) | 32.88 ± 4.47 | 30.87 ± 3.25 | .000 |
| Male age (years) | 33.91 ± 4.74 | 32.58 ± 3.68 | .000 |
|
Total Gn quantity (IU/day) (for ovarian stimulation) |
2266.01 ± 896.72 | 2017.14 ± 808.04 | .000 |
| Number of eggs retrieved | 9.06 ± 6.22 | 12.41 ± 5.46 | .000 |
| Number of embryos transferred | 1.27 ± 0.45 | 1.36 ± 0.48 | .036 |
| HCG positive rate (%) | 62.70 (3210/5120) | 58.54 (72/123) | .346 |
| Clinical pregnancy rate (%) | 59.08 (3025/5120) | 53.66 (66/123) | .227 |
| Male BMI | 25.14 ± 3.56 | 25.34 ± 3.62 | .450 |
| Semen volume (mL) | 3.77 ± 1.56 | 3.91 ± 1.70 | .255 |
| Sperm concentration (104/mL) | 60.27 ± 29.39 | 52.49 ± 28.54 | .001 |
| Sperm motility rate (%) | 53.00 ± 14.41 | 43.21 ± 15.06 | .000 |
| DNA fragmentation (%) | 14.16 ± 8.66 | 18.20 ± 8.55 | .006 |
| Normal morphology rate (%) | 3.19 ± 2.37 | 2.83 ± 2.08 | .000 |
- Abbreviations: BMI, body mass index; Gn, gonadotropin doses; HCG, human chorionic gonadotropin.
Male patients undergoing IVF treatment abstained from ejaculation for 48 hours prior to semen collection. On the day of oocyte retrieval, semen was collected into a sterile cup in the early morning and kept at room temperature until liquefaction. Density gradient centrifugation was then performed, using high-density gradient liquid, low-density gradient liquid, and patient semen added sequentially to the centrifuge tube. After centrifugation at room temperature for 20 min (300 × g), the pellet was retained and washed with 0.5 mL of sperm-washing solution. The washed pellet was mixed with 0.3–0.5 mL of fertilization medium and incubated at 37°C for 30 min to allow capacitation before fertilization. Following capacitation, the upper layer of sperm was collected, counted, and added to the eggs at a concentration of 50,000 sperm per well. After 4–5 h of conventional IVF insemination, cumulus cells were removed using a denudation pipette with an inner diameter of 140 µm. Short-time fertilization was considered successful if the number of oocytes with two polar bodies (2PB) observed under the microscope was greater than or equal to half of the total number of mature oocytes. If the criteria were not met after extended observation, early RICSI was performed on oocytes with only one visible polar body. Fertilization status was reassessed the following day, with the presence of two pronuclei (2PN) indicating successful fertilization, whether through conventional IVF or ICSI. The remaining fertilization medium containing sperm was subsequently collected for further analysis in the conventional IVF group (Group IVF) or the RICSI group (Group RICSI). To minimize individual differences, 10 samples from each group were pooled into five mixed samples per group (subgroups IVF-1 to IVF-5 and subgroups RICSI-1 to RICSI-5) for lectin microarray analysis. The remaining samples were stored individually for further verification.
2.2 Sperm and sperm-derived exosomes collection
The sperm media was centrifuged at 200 × g for 5 min to separate the sperm from the culture media. The sperm pellet was collected and stored at −80°C. The supernatant was transferred to a new tube for exosome extraction using the Magenteh HiPure Exosome RNA Kit (R4319-02), following the manufacturer's instructions. Briefly, the supernatant was centrifuged at 2000 × g for 10 min to remove cell debris. The supernatant was then filtered through a 0.22 µm syringe filter. An exosome precipitation solution, equal to 0.5 times the sample volume, was added to the filtered supernatant, which was incubated at room temperature for 30 min. After incubation, the mixture was centrifuged at 10,000 × g for 10 min to pellet the exosomes. The pellet was resuspended in 200 µL of filtered 1× PBS, and the precipitation procedure was repeated. The final pellet was stored at −80°C for subsequent characterization and analysis.
2.3 Transmission electron microscope analysis
A transmission electron microscope (TEM) was employed to confirm the particles as exosomes in the sperm culture fluid. A 10 µL aliquot of the exosome solution was placed on a copper grid and negatively stained. After drying for 2 min under an incandescent bulb, the grid was examined and imaged using an H-7650 Hitachi transmission electron microscope (Hitachi).
2.4 Particle size analysis
A Flow Nano Analyzer (NanoFCM) was used to analyze the exosome solution for nanoparticle size and concentration, following a previously published protocol.15 Briefly, the sample stream was fully illuminated within the central area of the focused laser beam, achieving approximately 100% detection efficiency. To calibrate the sample flow rate, 100 nm orange FluoSpheres with a known particle concentration were used to determine the concentration of each exosome sample.
2.5 Flow cytometer analysis
The exosome solution (5–10 µL) was diluted with phosphate-buffered saline (PBS) into three different concentrations: 1/10, 1/100, and 1/1000. A Cytofex Flow Cytometer (Beckman) was first used to determine the number of extracellular vesicle particles and select the appropriate dilution where the average particle count was below 10,000. Subsequently, the sample was incubated with 1 µg of PE-anti-mouse/cat CD81 (104905, Biolegend) or APC-CD63 (143905, Biolegend) antibodies for 15 min at room temperature. Following incubation, the Cytofex Flow Cytometer was employed to assess the marker expression.
2.6 Total protein extraction and labeling
The total protein from sperms or exosomes was extracted using T-PER Reagent (Pierce Biotechnology Inc.) following the manufacturer's guidelines. Briefly, the sperm/exosome pellet was resuspended in an appropriate volume of T-PER Reagent containing protease inhibitors (10 µL per 1 mL of reagent), incubated on ice for 45 min, and homogenized. After centrifuging at 10,000 × g for 10 min, the supernatant was either used immediately or stored at −80°C. Protein concentration was measured using the BCA assay. The extracted protein was then labeled with Cy3 fluorescent dye (GE Healthcare) and purified using Sephadex G-25 columns per the manufacturer's instructions.
2.7 Lectin microarrays and data analysis
A lectin microarray containing 20 lectins with specific binding preferences for N- and O-linked glycans was constructed as previously described.16 Lectins were prepared according to the manufacturer's instructions and spotted onto epoxysilane-coated slides using a PersonalArrayer 16 (CapitalBio). Each lectin was printed in triplicate across three blocks per slide. PBS served as a blank control, unlabeled BSA as a negative control, and Cy3-labeled BSA was used as a location marker. The slides were incubated overnight at 50% humidity, dried in a vacuum at 37°C for 3 h, and washed with 1× PBST and 1× PBS before being blocked with 2% BSA for 1 h. Cy3-labeled protein, diluted in incubation buffer, was applied and incubated at 37°C for 3 h in a rotisserie oven. After washing and drying, the slides were scanned using a Genepix 4000B confocal scanner (CapitalBio) with the photomultiplier tube set at 70% and the laser power at 100%. Images were captured at 532 nm using the Cy3 fluorescence detection channel. Background values were subtracted from each data point, and data points falling below the average background ± 2 standard deviations (SD) were excluded. The median of valid data points for each lectin was normalized against the sum of medians from all valid data points within the block. For each sample, three replicate slides were analyzed, and the normalized median values from nine replicate blocks were averaged, with the SD calculated. To assess changes in protein glycosylation levels, normalized data from experimental groups were compared to controls, and fold changes (FCs) of RICSI versus IVF were calculated. FCs ≥1.5 or ≤0.67 indicated upregulation or downregulation of specific glycans. Differences between datasets were evaluated using paired Student's t-tests in SPSS Statistics 19. Further hierarchical clustering analysis (HCA) was performed on the data using Expander 6.0 (http://acgt.cs.tau.ac.il/expander/).
2.8 Sperm microarray, exosome microarray, and data analysis
For individual validation of lectin microarray results, 18 samples from conventional IVF patients and 30 samples from RICSI patients were collected. Due to the limited sample quantities, three samples from each group were pooled into a single EP tube (resulting in six samples for the IVF group and 10 samples for the RICSI group). Sperm and sperm-derived exosomes from both groups were spotted onto the microarray slide to make sperm microarray and exosome microarray using a pipette, with each spot containing 0.2 µL of sample and three spots per sample.
After spotting, the slides were dried at room temperature for 1 h, then fixed with 4% paraformaldehyde for 10 min. Meanwhile, the lectins were labeled with Cy3 fluorescent dye and purified using Sephadex G-25 columns according to the manufacturer's instructions. The paraformaldehyde was removed by washing the slides with PBS for 5 min, three times. The slides were blocked with 4% BSA for 1 h, washed again with PBS, and incubated overnight at 4°C with Cy3-labeled lectins (DBA for sperm samples and Jacalin for sperm-derived exosome samples) and β-Actin Mouse Monoclonal Antibody (Catalog # 66009-1-Ig, Proteintech). After overnight incubation, the slides were washed three times with PBS (5 min each) and incubated with Cy5-labeled goat anti-mouse secondary antibody (Catalog # 1030-15, SouthernBiotech) for 1 h at room temperature. The slides were then washed with PBS and scanned using a Genepix 4000B confocal scanner with 70% photomultiplier tube and 50% laser power settings. Images were analyzed at 532 nm for Cy3 and 635 nm for Cy5 using Genepix 3.0 software. Data points below the average background ± 2 SD were excluded, and the median of effective data points for each spot was calculated. Medians within the same group were expressed as mean ± SD, and differences between the IVF and RICSI groups were tested using Student's t-test in SPSS Statistics 19.
2.9 Fluorescence immunohistochemistry
Sperm from IVF and RICSI groups were spotted onto the slide using a pipette, with each spot containing 0.2 µL of sample. After spotting, the slides were dried and fixed with 4% paraformaldehyde for 10 min. The fixed sperms were permeabilized in ice-cold PBS supplemented with 1% Triton X-100 at 4°C for 10 min and rinsed twice in PBS. Prior to incubation with the labeled lectin, the fixed sperms were blocked with the blocking buffer (PBS supplemented with 4% BSA) at 37°C for 1 h. Following that the sperms were incubated with the Cy3-labeled lectin and β-actin mouse monoclonal antibody diluted at a proper concentration with the blocking buffer overnight at 4°C in the dark. After washing, sperms were incubated with Cy5-labeled goat anti-mouse secondary antibody for 1 h at room temperature. Then, they were further stained with 1 µg/mL of DAPI (Roche) in PBS for 10 min and a final rinse was performed. A laser scanning confocal microscope Leica STELLARIS 5 (Leica Microsystems) was used to collect the images with the merge channels of Cy3, Cy5, and DAPI.
2.10 Sperm motility detection
RICSI sperm samples were incubated with varying amounts of IVF exosomes for 30 min. Following incubation, sperm motility was assessed using a computer-assisted sperm analysis system (Hunan Suijia Medical Technology Co., Ltd). Each sample was registered by verifying patient information and recording relevant details. Specimens were stored at 36°C ± 1°C and thoroughly mixed. The color and viscosity of each sample were checked following the guidelines outlined in the World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. A 10 µL aliquot was then loaded into a Makler chamber, placed on the microscope stage, and analyzed through the “Motility & Concentration” interface. The automated analysis was followed by a manual review to correct potential errors, such as bubbles or debris. If necessary, re-sampling was performed to ensure reliable measurements. Any flagged anomalies were marked for manual inspection, and adjustments, such as adding or reclassifying data points, were made as needed to ensure accurate and consistent results.
3 RESULTS
3.1 Comparison of clinical indicators of couples in the IVF and RICSI groups
In 2023, a total of 11,903 IVF-ET cycles were performed at Northwest Women and Children's Hospital, affiliated with Xi'an Jiaotong University. Among these, 8678 out of 8861 cycles achieved successful fertilization through conventional IVF, yielding a success rate of 97.9%. Meanwhile, 187 cycles necessitated RICSI due to either total fertilization failure (120 cycles, accounting for 64.2%) or partial fertilization failure (67 cycles, accounting for 35.8%), resulting in an incidence rate of 2.11%. Of these RICSI cases, 183 achieved successful fertilization, with a success rate of 97.9%. The clinical data for couples undergoing conventional IVF and RICSI cycles are presented in Table 1. The analysis showed that couples in the RICSI group had a significantly longer duration of infertility, but both the wives and husbands were significantly younger compared to the IVF group. For wives, the total Gn quantity, an indicator of ovarian function, was significantly lower, while the number of oocytes retrieved and embryos transferred was significantly higher in the RICSI group than in the IVF group. Despite these differences, the HCG positivity rate and clinical pregnancy rate were similar between the two groups. For husbands, there was no significant difference in body mass index (BMI) or semen volume between the two groups. However, sperm concentration, motility, and normal morphology rates were significantly lower, while DNA fragmentation rates were significantly higher in the RICSI group compared to the IVF group. Despite these sperm quality differences, conventional IVF procedures were uniformly applied because all cases met traditional IVF criteria. During fertilization, sperm was processed according to the previously described method, achieving a viability rate of over 95%, with comparable sperm concentration and motility rates between the two groups.
3.2 Characterization of sperm-derived exosomes
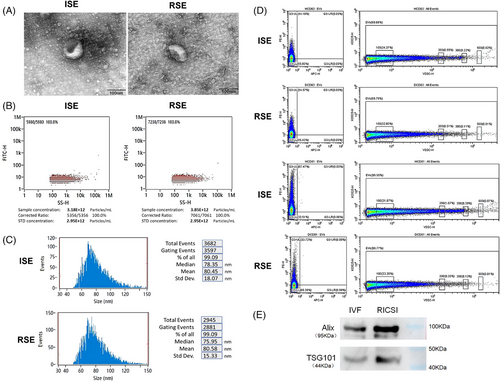
TEM analysis of sperm-derived exosomes (SE) at 60,000× magnification and 100 kV revealed a saucer-like structure in both groups (Figure 1A). To estimate particle concentration and size, NanoFCM size distribution analysis was performed. The concentrations of IVF sperm-derived exosomes (ISE) and RICSI sperm-derived exosomes (RSE) were 3.18 × 10¹2/mL and 3.85 × 10¹2/mL, respectively (Figure 1B). The mean diameters of ISE and RSE were 80.45 ± 18.07 and 80.58 ± 15.33 nm, respectively (Figure 1C). There was no significant difference in exosome concentration and size between the two groups. Additionally, CytoFLEX flow cytometer analysis revealed that 44.10% and 67.47% of ISE were marked with CD63 and CD81, while 54.57% and 33.72% of RSE were marked with CD63 and CD81, respectively (Figure 1D). Notably, higher CD63 and lower CD81 expression levels were observed in RSE compared to ISE.
3.3 Glycopattern of sperms and sperm-derived exosomes
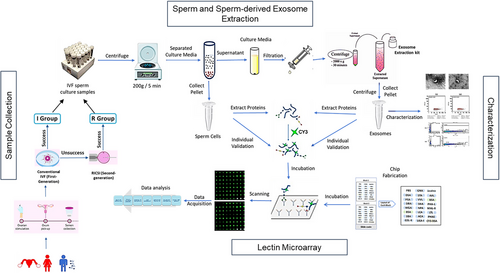
The glycopatterns of total proteins from sperms and exosomes were detected using high-throughput lectin microarrays. The workflow used in this study is shown in Figure 2. The binding curves for each lectin were generated by measuring signal intensity across a range of total protein quantities. Cy3-labeled total proteins from IVF sperm (IS) and ISE were applied to the lectin microarrays at concentrations of 2, 4, and 6 µg each (Figures S1 and S2). Ultimately, 4 µg of sperm proteins and 6 µg of exosomal proteins were used to ensure that all binding signals fell within the approximate linear binding range for each lectin.
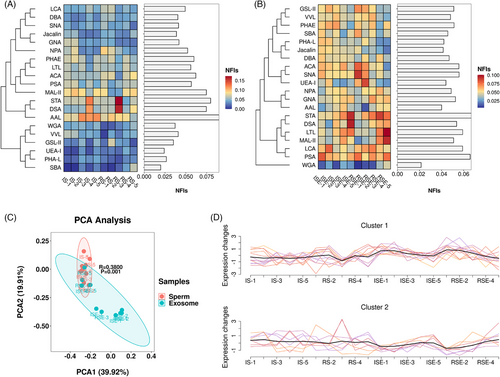
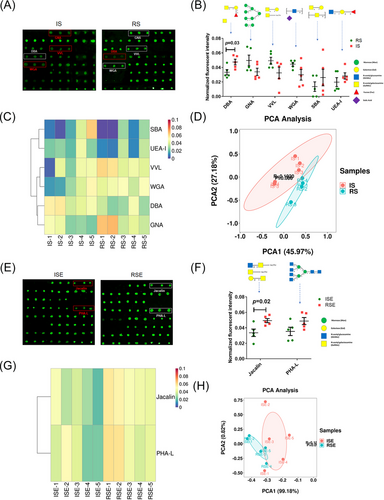
The optimal quantity of glycoproteins from sperm and sperm-derived exosomes was determined using lectin microarrays. The sugar-binding specificities and normalized fluorescent intensities (NFIs) of 20 lectins for all sperms and exosomes are summarized in Tables S1 and S2. For most sperm samples, the Fucα1-3(Galβ1-4)GlcNAc and Fucα-1,6GlcNAc (core fucose) binder AAL, along with the α-tetramers and trimers of GlcNAc binder STA, showed the strongest signals, while the (GalNAc)n, α- or β-linked terminal GalNAc binder SBA and the Fucα1-2Galβ1-4Glc(NAc) binder UEA-I showed the weakest signals (Figure 3A). In exosomes, the α-tetramers and trimers of GlcNAc binder STA and the Fucα-1,6GlcNAc, α-D-Glc, and α-D-Man binder PSA exhibited the strongest signals, while the (GlcNAc)n and multivalent Sia binder WGA showed the weakest signals (Figure 3B). The average NFI levels of each lectin across all samples are displayed as a bar graph alongside a heatmap. Principal component analysis (PCA) of the 20 lectins in all samples revealed significant differences between sperm and exosomes (Figure 3C). Based on the observed trends, these 20 lectins were categorized into two clusters: Cluster-1, which includes 12 lectins (e.g., Jacalin and PSA) that showed higher intensities in exosomes compared to sperm, and Cluster-2, which includes eight lectins (e.g., GNA and DSA) that exhibited lower intensities in exosomes compared to sperm (Figure 3D).
3.4 Differences in glycan expression on proteins of sperm and sperm-derived exosomes between IVF and RICSI groups
To investigate glycopattern differences between the IVF RICSI and groups, the NFIs of each lectin in the RICSI group were compared to those in the IVF group using a two-tailed Student's t-test, based on fold-changes (FCs) between paired samples (RS vs. IS and RSE vs. ISE). Lectins were considered significantly different if their FC was greater than 1.40 or less than 0.71, with a p-value less than .05. As shown in the lectin microarray image (Figure 4A), DBA, a binder of αGalNAc, Tn antigen, GalNAcα1-3(Fucα1-2)Gal (blood group A antigen), exhibited a significantly lower NFI in RS compared to IS (FC = 0.70, p = .003). Additionally, GNA (an α1-3Man and high-mannose binder), VVL (a terminal GalNAc and GalNAcα-Ser/Thr (Tn) binder), and WGA (a multivalent Sia and (GlcNAc)n binder) showed increased NFIs, while SBA (a β- or α-linked terminal GalNAc and (GalNAc)n binder) and UEA-1 (a Fucα1-2Galβ1-4GlcNAc binder) showed decreased NFIs in RS versus IS (Figure 4B). However, t-test analysis indicated no significant differences for these lectins. Heatmap and PCA of these six altered lectins significantly distinguished the RS group from the IS group (Figure 4C,D). For sperm-derived exosomes, as shown in the lectin microarray image (Figure 4E), Jacalin, a binder of Galβ1-3GalNAcα-Ser/Thr (T), GalNAcα-Ser/Thr (Tn), GlcNAcβ1-3GalNAcα-Ser/Thr (Core3), and sialyl-T, exhibited significantly increased NFIs in RSE compared to ISE (FC = 1.47, p = .02). Additionally, PHA-L, a tetra-antennary complex-type N-glycan binder, showed slightly higher NFIs in RSE compared to ISE (Figure 4F). The heatmap and PCA of these two altered lectins significantly distinguished the RSE group from the ISE group (Figure 4G,H).
3.5 Difference in expression of glycans on proteins between sperms and sperm-derived exosomes in each group
To examine the variation in glycopatterns between sperm and sperm-derived exosomes in the RICSI and IVF groups, the NFIs of each lectin from sperm and exosomes within the same group were compared using a two-tailed Student's t-test based on fold-changes (FC) between pairs (ISE vs. IS and RSE vs. RS). Lectins with FC values greater than 1.50 or less than 0.67, and p-values below .05, were considered significantly different. In the IVF group, GNA, a high-mannose and Manα1-3Man binder, showed significantly higher NFIs in ISE compared to IS (FC = 1.58, p = .03), while AAL, a Fucα1-6GlcNAc (core fucose) and Fucα1-3(Galβ1-4)GlcNAc binder, showed significantly lower NFIs (FC = 0.48, p = .003) (Figure 5A). Additionally, the tetra-antennary complex-type N-glycan binder PHA-L, the α- or β-linked terminal GalNAc and (GalNAc)n binder SBA, and the Fucα1-2Galβ1-4GlcNAc binder UEA-I displayed higher NFIs in ISE compared to IS, while DSA, a β-D-GlcNAc, (GlcNAcβ1-4)n, and Galβ1-4GlcNAc binder showed lower NFIs. However, these differences were not statistically significant (Figure 5B). Heatmap and PCA analyses of the six altered lectins revealed significant differences between ISE and IS samples (Figure 5C,D).
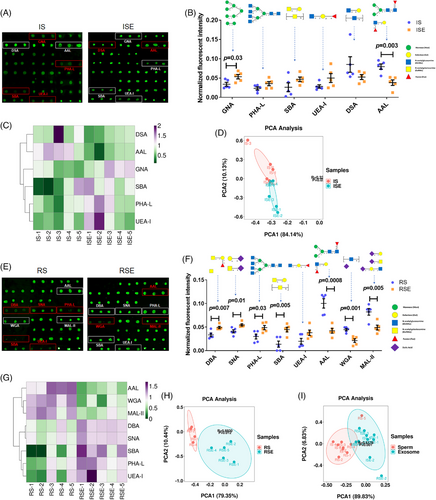
In the RICSI group, the tetra-antennary complex-type N-glycan binder PHA-L (FC = 1.63, p = .03) and the α- or β-linked terminal GalNAc and (GalNAc)n binder SBA (FC = 3.28, p = .005) showed significantly higher NFIs in RSE compared to RS. Conversely, AAL (FC = 0.42, p = .0008), a multivalent Sia and (GlcNAc)n binder WGA (FC = 0.48, p = .001), and MAL-II, a Siaα2-3Galβ1-4GlcNAc/Glc binder (FC = 0.59, p = .005), exhibited significantly lower NFIs in RSE versus RS (Figure 5E). Additionally, UEA-I showed higher NFIs in SSE compared to SS, but the difference was not statistically significant. The DBA (an αGalNAc, Tn antigen, and GalNAcα1-3(Fucα1-2)Gal binder) and SNA (a Sia2-6Gal/GalNAc binder) demonstrated slightly higher NFIs with significant differences in RSE versus RS (Figure 5F). Heatmap and PCA analyses of the eight altered lectins revealed significant differences between RSE and RS samples (Figure 5G,H).
Among all differentially expressed glycans, those recognized by PHA-L (tetra-antennary complex-type N-glycan), SBA (α- or β-linked terminal GalNAc and (GalNAc)n), and UEA-I (Fucα1-2Galβ1-4Glc(NAc)) showed higher expression in all exosomes compared to sperm. In contrast, glycans recognized by AAL (Fucα1-6GlcNAc [core fucose] and Fucα1-3(Galβ1-4)GlcNAc) exhibited lower expression. PCA of these four lectins indicated a significant difference between sperm and sperm-derived exosomes (Figure 5I).
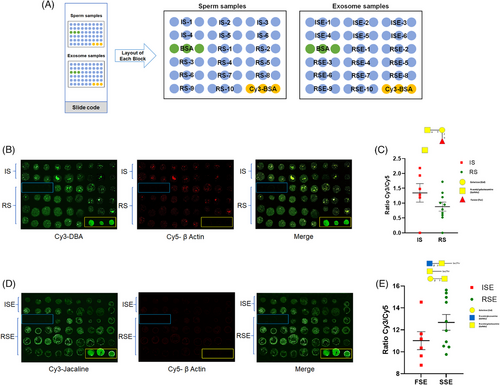
3.6 Individual validation using sperm and exosome microarrays
Sperm and sperm-derived exosomes from individual samples were spotted onto slides and incubated with specific lectins to validate the expression of certain glycans. The layout of the sample microarray is shown in Figure 6A. Based on the lectin microarray results, glycans recognized by DBA and Jacalin were differentially expressed in sperm and exosomes, respectively, between the two groups. Therefore, Cy3-labeled DBA was used for incubation with the sperm microarray, and Cy3-labeled Jacalin was used for incubation with the exosome microarray. To ensure accurate comparison between samples from both groups, Cy5-labeled beta-actin was used as an internal reference to normalize the glycan expression in each sample by calculating the ratio of Cy3 (lectins) to Cy5 (beta-actin). On the sperm microarray (Figure 6B), DBA showed a decreased Cy3/Cy5 ratio, indicating that αGalNAc, Tn antigen, and GalNAcα1-3(Fucα1-2)Gal (blood group A antigen) were expressed at lower levels in RS compared to IS (Figure 6C). On the exosome microarray (Figure 6D), Jacalin showed an increased Cy3/Cy5 ratio, suggesting that Galβ1-3GalNAcα-Ser/Thr (T), GalNAcα-Ser/Thr (Tn), GlcNAcβ1-3GalNAcα-Ser/Thr (Core3), and sialyl-T (ST) were expressed at higher levels in RSE compared to ISE (Figure 6E). Although these changes were not statistically significant, the trends in glycan expression detected by DBA and Jacalin in the RICSI versus IVF groups were consistent with the results from the lectin microarray.
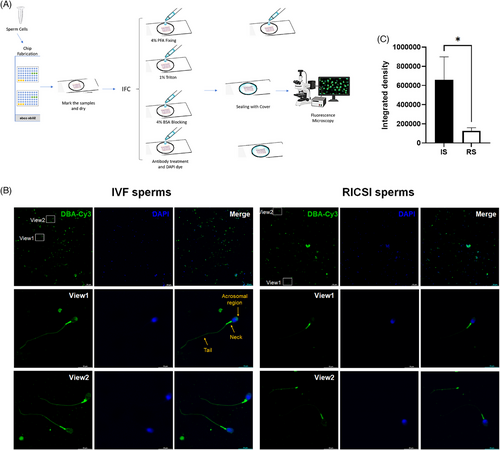
3.7 Expression and distribution of glycans bound by DBA in sperms from two groups
To further examine the expression and distribution of DBA-recognized glycans in sperm, Cy3-DBA staining was applied to immobilized sperm on slides using lectin histochemistry (Figure 7A). The results showed strong DBA binding to the neck region and moderate binding to the acrosomal region, the plasma membrane of the sperm head, and the tail in IVF sperm. However, the binding intensity was reduced in these same regions in RICSI sperm (Figure 7B). The integrated density values obtained through ImageJ analysis were significantly lower in RICSI sperm compared to IVF sperm (Figure 7C).
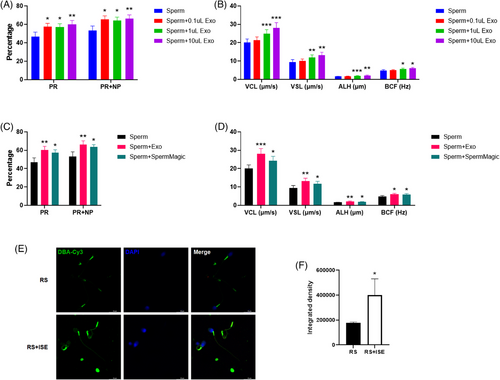
3.8 IVF exosomes enhance the motility of RICSI sperm
As exosomes mediate intercellular communication and transfer essential biological information, we aimed to explore whether IVF exosomes could improve the motility of RICSI sperm. RICSI sperm samples were stored at 36°C ± 1°C for 2 days before being treated with 0.1, 1, and 10 µL of IVF exosomes (concentration: 3.9 × 10¹¹ particles/mL). A total of eight RICSI sperm samples were used to assess motility. The results showed that IVF exosomes significantly enhanced progressive motility (PR) and total motility (PR + non-progressive [NP]) in a dose-dependent manner (Figure 8A). Additionally, they promoted key motility parameters in a dose-dependent manner also, including curvilinear velocity (VCL), straight-line velocity (VSL), lateral amplitude (ALH), and beat cross frequency (BCF) (Figure 8B). To further validate this enhancement, we compared the effects of exosome treatment with SpermMagic (Reprobiotech Corporation), a known motility enhancer. Both treatments significantly improved sperm motility across all indicators (Figure 8C,D). However, exosome treatment yielded superior results, demonstrating a more substantial improvement in motility parameters. Further DBA staining revealed a significant increase in the expression of αGalNAc, Tn antigen, and GalNAcα1-3(Fucα1-2)Gal (blood group A antigen) in the neck and tail of RICSI sperm after incubation with IVF-derived exosomes (Figures 5E and 8F).
4 DISCUSSION
The distinct glycan profiles of exosomes and small extracellular vesicles (sEVs), determined by their host cell types and subpopulations, play pivotal roles in biological processes such as cancer occurrence, metastasis, and microenvironmental regulation.16, 17 Lectin microarrays have proven to be an effective method for profiling exosome glycan structures, especially when the low abundance of exosomes makes mass spectrometry challenging. This technique offers high-throughput and sensitive detection of glycans, making it a powerful tool for glycomic analysis in cancer biomarker discovery and disease diagnosis. In our previous study, we used a lectin microarray to reveal differentially expressed glycans in cells and exosomes related to gastrointestinal cancer,14b which provided valuable insights for biomarker discovery. In this study, we applied the lectin microarray to generate a full-scale glycan fingerprint of sperm and sperm-derived exosomes related to conventional IVF and RICSI, aiming to identify specific biomarkers and explore the underlying mechanisms of glycosylation in fertilization. The study included 50 patients per group (IVF and RICSI), with 10 individual samples pooled into five mixed samples per group for lectin microarray analysis to reduce noise and ensure consistent glycosylation profiling. Individual samples were retained for validation experiments to support findings. Pooling was chosen to ensure sufficient protein quantities for reliable lectin microarray detection while balancing variability and representativeness. Exosome isolation and characterization, challenging due to their nanoscale size and sample complexity, utilized a commercial kit and rigorous protocols, including filtration, precipitation, and size-based characterization, to enhance purity and specificity for downstream analyses.15, 18
Chromosomal polymorphisms19 and sperm DNA damage20 are significant contributors to IVF failure, impairing spermatogenesis, reducing sperm quality and fertilization rates, and increasing the risk of embryonic aneuploidy, thereby negatively impacting ART success. However, in some clinical cases, fertilization failure in conventional IVF was followed by successful fertilization after transitioning to ICSI, with resulting zygotes undergoing normal division. This suggests that significant chromosomal abnormalities in gametes are unlikely to be the primary cause. Instead, the issue likely lies in sperm–egg recognition21 or subtle abnormalities that contribute to unexplained infertility. According to 2023 statistics in this study, the HCG-positive rate and clinical pregnancy rate were similar between the two groups, indicating that the women's ability to conceive and the health status of the embryos were comparable. For males, although both groups met the criteria of high-quality sperm with a motility rate exceeding 95% and equal sperm numbers per well during fertilization, the unprocessed semen in the RICSI group had significantly lower sperm concentration, motility, and normal morphology rates, and higher DNA fragmentation, compared to the IVF group. Given the challenges in obtaining oocytes and the glycan-rich nature of sperm surfaces, this study focuses on analyzing sperm glycan alterations in RICSI versus IVF. Additionally, sperm-derived exosomes, which partially reflect host cell composition and regulate the sperm microenvironment, remain understudied. Investigating their glycan profiles is essential for understanding fertilization mechanisms and identifying potential biomarkers or therapeutic targets to improve ART outcomes.
Fucose on sperm plays a crucial role in sperm–egg interactions across species, such as oysters, bovines, and Drosophila, by facilitating binding to egg surfaces, with sperm-associated fucose-binding lectins and α-L-fucosidases mediating species-specific recognition and fertilization.22 Additionally, fucose plays an essential role in sperm capacitation and fertilization, undergoing redistribution during human sperm capacitation, particularly in the acrosomal region.23 It is also involved in triggering the acrosome reaction (AR) in mammals by activating signaling pathways in sperm.24 Furthermore, seminal plasma glycoproteins, such as clusterin and galectin-3-binding protein, are heavily fucosylated and play a role in immune response processes and reactome pathways.25 In this study, we found that Fucα1-3(Galβ1-4)GlcNAc and Fucα-1,6GlcNAc (core fucose), recognized by AAL, were the most abundant in human sperm, while α-D-Man, Fucα-1,6GlcNAc (core fucose) recognized by LCA, and Fucα1-2Galβ1-4GlcNAc and Fucα1-3(Galβ1-4)GlcNAc recognized by LTL were moderately expressed, with Fucα1-2Galβ1-4Glc(NAc) recognized by UEA-I being the least expressed. In exosomes, fucose with different linkages was consistently moderately expressed. These findings suggest that different fucose linkages in sperm may play distinct roles, and their expression patterns in exosomes differ accordingly.
Dolichos biflorus agglutinin (DBA) is a lectin that recognizes the glycan epitope α-N-acetylgalactosamine (GalNAc). In reproductive studies, DBA binds to glycan residues on the surface of gonocytes and spermatogonia, contributing to the regulation of cell adhesion, self-renewal, and germ cell potential. Its binding patterns help identify sugars involved in sperm maturation, capacitation, and oocyte recognition, as seen in species like horses and rats.26 In buffalo, bovine, and other species, DBA has been instrumental in isolating and enriching type A spermatogonia, facilitating the identification of spermatogonial stem cells (SSCs) for in vitro culture and transplantation experiments.27 Early studies also suggested that fluorescein isothiocyanate (FITC)-conjugated peanut agglutinin (PNA), soybean agglutinin (SBA), or DBA could bind to the acrosomal region of a few sperm from the toad Bufo japonicus.28 In this study, DBA showed significantly lower NFI in sperm from the RICSI group compared to conventional IVF. Sperm microarray and lectin fluorescent histochemistry further revealed that the expression of αGalNAc, Tn antigen, and GalNAcα1-3(Fucα1-2)Gal (blood group A antigen), recognized by DBA, was significantly reduced in individual sperm samples from the RICSI group compared to the IVF group, particularly in the acrosomal region of head, neck, and tail. Given the role of exosomes in intercellular communication and signal transduction, we investigated whether IVF-derived exosomes could enhance the motility of RICSI sperm. Our results showed that IVF exosomes significantly improved key motility parameters of RICSI sperm in a dose-dependent manner by upregulating DBA-recognized glycans on their surface. These findings suggest that DBA-recognized glycans on human sperm are essential for motility and fertility.
The T and Tn antigens are truncated O-glycans, and their abnormal expression is a hallmark of various cancers, including breast, gastric, pancreatic, and colon cancers. These antigens are typically masked in normal cells but become exposed in cancerous cells due to altered glycosylation processes. A recent study revealed that the Tn antigen promotes tumor growth and lung metastasis in triple-negative breast cancer and contributes to the creation of an immunosuppressive environment. Jacalin, a lectin that binds to Galβ1-3NAcGalα-Ser/Thr (T), NAcGalα-Ser/Thr (Tn), NAcGlcβ1-3NAcGalα-Ser/Thr (Core 3), and sialyl-T, was found in our lectin microarray results to exhibit significantly increased NFIs in sperm-derived exosomes from RICSI compared to ISE, indicating that T/Tn antigens and Core 3 are highly expressed in RICSI-derived exosomes compared to those from conventional IVF. Recent research highlights that extracellular components of semen, particularly glycoproteins, influence the female immune response, promoting immune tolerance crucial for embryo implantation and pregnancy. Seminal plasma glycoproteins, including those carrying T/Tn antigens and Lewis-type glycans, interact with maternal immune cells, potentially modulating immune responses to support fertilization and pregnancy. Although studies on T/Tn antigens in exosomes are limited, it is speculated that these glycans may play roles in sperm function and fertilization.
In conclusion, DBA-recognized glycans were significantly downregulated in sperm, while Jacalin-recognized glycans were significantly upregulated in exosomes in the RICSI group compared to the IVF group. Glycans bound by PHA-L, SBA, and UEA-I were highly expressed, whereas those bound by AAL were less expressed in exosomes compared to sperm in both groups. Additionally, DBA binding was primarily localized to the neck, acrosomal region, plasma membrane of the sperm head, and tail in IVF sperm, but was significantly reduced in RICSI sperm. Functionally, IVF-derived exosomes enhanced key motility parameters of RICSI sperm in a dose-dependent manner by upregulating DBA-recognized glycans on their surface. These findings offer valuable insights into biomarker identification, optimization of IVF therapeutic strategies, and the role of glycosylation in sperm and exosome-related infertility, while also suggesting the potential of exosome-based therapy to improve RICSI sperm motility.
ACKNOWLEDGMENTS
The authors are grateful to Xiaofei Wang, Liying Liu, and Wen Li in the Biomedical Experimental Center of Xi'an Jiaotong University, Xi'an, China and Baochang Lai in the Cardiovascular Research Center, School of Basic Medical Sciences, Xi'an Jiaotong University, Xi'an, China for their technical supports, and sincerely thank all the participants.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.