A lasting antibacterial, pro-angiogenic, and pro-osteogenic zirconium-based bulk metallic glass for dental implants
Feifei Wang, Yunshu Wu, and Fu Zheng contributed equally to this study.
Abstract
Bacterial infection and mismatched mechanical properties are important factors that increase the risk of dental implant failure. However, zirconium (Zr)-based bulk metallic glasses (BMGs) can have both high strength and low modulus, as well as good biocompatibility, due to their unique atomic arrangement structure. Based on these common characteristics, different elemental compositions can endow zirconium-based amorphous alloys with different properties. Here, we present a Zr-based BMG containing silver (Ag) with good amorphous process ability, exhibiting lasting antibacterial, proangiogenic, and pro-osteogenic properties. This newly developed Zr61Cu23Al12Ti2Ag2 (at.%) BMG has higher strength and lower modulus than pure Titanium (Ti). Furthermore, it could exert antibacterial effects through both contact inhibition and metal ion sterilization. And this antibacterial property could last over 3 months. The systematically in vitro and in vivo results thus demonstrate the advantages and application potentials of Zr-based BMG as a highly promising oral implant material for dental implantation.
1 INTRODUCTION
Implant treatment has been widely accepted as a predictable option for most clinical situations seen in routine clinical practice.1 Titanium (Ti) is widely used in implants due to its moderate mechanical properties and good biocompatibility.2-4 However, its elastic modulus mismatch with natural bone can cause stress shielding, affecting implant stability. Additionally, Ti's lower strength compared to other materials like Co-Cr alloys and stainless steel increases the risk of fractures, especially in narrow diameter implants.5, 6 Meanwhile, titanium lacks inherent osteoinductive properties, which can possibly result in suboptimal osseointegration, particularly in cases with low bone density or osteoporosis,7 leading to concerns about the prolonged treatment duration necessary for successful implant integration. More importantly, Ti lacks intrinsic antimicrobial properties, raising the risk of postimplantation infections. Previous reports indicate that the incidence rate of peri-implantitis has reached as high as 51%.8 Therefore, it remains challenging to develop the next generation of dental implants with lasting antibacterial ability, high osseointegration capacity, and suitable mechanical properties.
Bulk metallic glasses (BMGs), due to their atomically disordered structure and lack of defects like grain boundaries and dislocations, combine the high strength of metals with the low elastic modulus of bioactive glasses.9 Among them, zirconium (Zr)-based BMG has emerged as the widely studied metallic biomaterials due to its good biocompatibility, corrosion resistance, and adjustable mechanical properties.10 For example, Ida et al. reported that the Zr70Ni16Cu6Al8 BMG was conducive to adhesion, proliferation of rat bone marrow mesenchymal cells, and had shown better stability, bone integration rate, and bone integration area compared to Ti and Ti alloys.11 Furthermore, nickel (Ni)-free Zr50+xCu35-xAl7Nb5Pd3 (x = 0, 5) BMG had been designed and fabricated, and the results demonstrated that the Zr-Cu-Al-Nb-Pd BMGs had good in vitro biosafe and high corrosion resistance in simulated body fluid (SBF) environments.12 Further, Zr-based BMGs possess the unique capability to tailor their properties by adjusting their elemental composition,13 yet, the presence of elements like Ni, Be in Zr-based BMGs may cause allergic reaction or other adverse effect.11, 14-16 Hence, the current challenge lies in identifying Zr-based BMG compositions with biocompatible elements that possess good amorphous processability, matched mechanical properties, and high osseointegrated capacity required for dental implants. Recent studies on the antibacterial properties of Zr-based BMGs have shown promising developments. Du et al. found that Zr-based BMG samples displayed notable antibacterial activity against both Escherichia coli and Staphylococcus aureus via laser-induced periodic surface structures.17 However, there is currently a lack of antibacterial experiments targeting oral pathogens, and continuous monitoring of the antibacterial properties of Zr-based BMG is lacking.
Silver (Ag) is currently the most potent metal element with antibacterial properties18 for wide biomedical applications. Liu et al. reported that titanium implants coated with Ag nanoparticles displayed considerable antibacterial effectiveness against common oral pathogens, such as Streptococcus mutans.19 Moreover, some researchers have proposed enhancing the antibacterial performance of titanium alloys by incorporating antibacterial elements such as Cu and Ag. Shirai demonstrated the antibacterial activity of Ti-Cu alloy containing 1 wt% Cu,20 and Zhang proved that the precipitation of the Ti2Ag phase in Ti-Ag alloy was the main reason for its antibacterial performance.21
Yang et al. investigated the impact of Ag substitution for Ti in Zr56Cu24Al9Ni7Ti4-xAgx (x = 0, 1, 2, 3, and 4 at. %) BMGs on their glass-forming ability (GFA), thermal stability, and mechanical properties. They found that the alloys with x = 2 exhibited the highest ultimate fracture strength and plastic strain.22 Meanwhile, previous studies had shown that Zr-Ti-Cu-Al BMGs had low elastic modulus and excellent corrosion resistance, making them highly promising for biomedical implants and medical devices.23-25 Based on the above research, it is possible that introducing a portion of Ag into Zr-Ti-Cu-Al system may lead to the development of a promising new material for dental implant applications. Moreover, it remains unknown whether the blending Ag can also contribute to lasting antibacterial effects while keeping the appropriate mechanical properties.
Here, we developed a novel amorphous alloy system Zr61Cu23Al12Ti2Ag2 (at.%) (Zr-BMG) and evaluated its physicochemical properties, biocompatibility, antibacterial activity, osteogenic potential, and angiogenic capabilities, aiming to explore its potential as a material for oral implant applications. This formulation intentionally excluded elements (such as Ni, Be, Fe) that were considered unsuitable for use in oral implant systems. In ensuring both excellent amorphous forming ability and system stability, Ag had been incorporated to endow the material with antimicrobial properties, resulting in a sustained antibacterial effect lasting over 3 months. Concurrently, experiments had been meticulously designed in alignment with the specific requirements of dental implants to validate the performance of this new material.
2 MATERIALS AND METHODS
2.1 Materials preparation and characterization
Zr61Cu23Al12Ti2Ag2 was prepared by arc-melting (Institute of Physics CAS) of pure elements with a minimum 99.95% purity in an argon gas protective atmosphere, forming cylindrical samples with a diameter of 5 mm and rectangular samples with a width of 16 mm. Grade IV Ti was used for a comparison substrate and all metals were purchased from Beijing Jiaming Platinum Industry Nonferrous Metals Co., Ltd.
The phase composition of the sample was verified using an X-ray diffraction (XRD, D8 Advance, Bruker) instrument and a transmission electron microscope (TEM, JEM 2100PLUS, JEOL). Then, they were tested using a differential scanning calorimetry (DSC, DSC 404 F3, NETZSCH) instrument. Chemical compositions of material surfaces of the samples were analyzed using scanning electron microscopy (SEM, S-4800, HITACHI) and energy dispersive X-ray spectroscopy (EDS, S-4800, HITACHI). The surface microstructure was analyzed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250X, ThermoFisher Scientific). A monochromated Al Kα X-ray source of 1486.6 eV was used. The narrow spectra were further deconvoluted with Thermo Advantage software.
Material density was measured by micromeritics (AccuPyc II 1345). Compression test was carried out using a universal testing machine (Instron 5967) with a strain rate of 1×10−3 s−1 at room temperature. Ti and Zr-BMG samples (Ø5 mm) were cut and tested for mechanical properties using nanoindentation with a triangular diamond indenter (TI 950, Hysitron). Ti and Zr-BMG samples were uniformly polished to a 5000 grit and their wettability was characterized using static contact angle measurements with a surface contact angle meter (DSA 100E, KRÜSS). Tests were conducted at 20°C using SBF (Beyotime) as the reference fluid. For the above experiments, three parallel samples were set for each group.
The biocorrosion behaviors of the Zr-BMG and Ti were analyzed in an electrochemical workstation (Versa STAT 3F) in the SBF at 37°C. After cleaning and air drying, samples were then immersed in 200 µL SBF for varying durations: 1 day, 1 week, 2 weeks, 4 weeks, 8 weeks, and 12 weeks. A blank SBF control was also maintained. The ion concentrations in the SBF were analyzed using inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer). For the above experiments, three parallel samples were set for each group.
2.2 Biocompatibility measurements
Approved by the Medical Ethical Commission of Peking University School and Hospital of Stomatology (PKUSSIRB-202169161), human jawbone marrow mesenchymal stem cells (hJBMMSCs) were extracted from nonsmoking patients aged 18–45 undergoing orthognathic surgery and cultured as described in our previous research.26
The cell counting kit-8 (CCK-8, Dojindo Laboratories) was used to assess cell viability according to the manufacturer's instructions. Optical density (OD) values were then measured at 450 nm using an Infinite F50 microplate reader (Elx808, BioTek). The cytotoxicity of Zr-BMG and Ti samples on hJBMMSCs was assessed using a LIVE/DEAD cell kit (KeyGen Biotech) following the manufacturer's instructions. Samples were photographed under a fluorescence microscope (TCS-SP8 STED 3X, Leica).
hJBMMSCs were cultured on material samples in 96-well plates for 1, 4, and 24 h. Adherent cells were fixed with 4% paraformaldehyde (PFA, Solarbio) for 15 min at room temperature and then stained with DAPI (Sigma). Immunofluorescent images were acquired using a laser scanning confocal microscope (Eclipse Ti2, Nikon). Cell counts were conducted in five randomly selected fields and analyzed using Image J software (National Institutes of Health).
After incubation on the surface of material samples for 24 h, cells were fixed in 4% PFA. Subsequently, they were exposed to a solution of 0.1% Triton-X100 (Sigma) for 10 min at room temperature. Staining was performed using TRITC Phalloidin (Yeasen) to visualize F-actin and DAPI (Sigma) for nuclear staining. Samples were examined under a fluorescence microscope (TCS-SP8 STED 3X, Leica) using a Z-axis layer. Three random fields per material were imaged across three replicates.
After incubation on the surface of material samples for 24 h, cells were fixed in 2.5% glutaraldehyde solution (Solarbio) overnight. After graded dehydration and drying, the specimens were gold sputter-coated for 35 s before examination under a scanning electron microscope (SEM, JSM-7900F, JEOL).
2.3 Antibacterial evaluation
In a sterile environment, S. mutans (UA159) was streak-plated on Mannitol Salt Agar (MSA) solid medium and cultured at 37°C with 5% CO2 for 48 h, while Porphyromonas gingivalis (P. gingivalis, ATCC 33277) was streak-plated on Tryptic Soy Agar (TSA) solid medium and incubated in an anaerobic environment (80% N2, 10% CO2, 10% H2) at 37°C for the same duration. 107 CFU/mL bacterial suspensions were used for the following experiments.
After sterilization, the materials were placed in a 96-well plate and exposed to S. mutans and P. gingivalis suspensions for 24 h. Dual staining with SYTO 9 and Propidium iodide (15 min, dark) allowed examination under a laser confocal microscope (TCS-SP8 STED 3X) to differentiate live and dead bacteria using 488 and 560 nm excitation wavelengths.
Sterilized materials were placed in a 96-well plate and inoculated with 100 µL bacterial suspensions of S. mutans and P. gingivalis. The XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay (KeyGEN BioTECH) was used to evaluate bacterial metabolic activity following the manufacturer's protocol.
After 24 h coculture with S. mutans and P. gingivalis, the materials were washed, fixed in 2.5% glutaraldehyde at 4°C overnight, dehydrated with ethanol, air-dried, affixed to the SEM stage, gold-coated, and examined under SEM (JSM-7900F) at 10 kV to observe bacterial morphology and distribution.
After 24 h of coculture, the material was washed, incubated with 2',7'-Dichlorodihydrofluorescein diacetate (DCFH-DA, Beyotime) for 30 min in the dark, and observed under a laser confocal microscope (TCS-SP8 STED 3X) at 488 nm. The collected culture medium was also incubated with DCFH-DA (1:500 final concentration), transferred to a microplate, and fluorescence intensity at 522 nm was measured using a microplate reader.
After coculturing the material with bacteria for 24 h, the original culture medium was collected in a sterile centrifuge tube and centrifuged at 5000 rpm for 10 min. The protein content released by the bacteria in the supernatant was tested with a bicinchoninic acid (BCA, Beyotime) assay kit and measured at 562 nm.
2.4 In vitro cell angiogenesis and osteogenic measurements
To investigate in vitro angiogenesis, HUVECs (Cellverse Bioscience Technology Co., Ltd.) were cultured in extracellular matrix (ECM) complete culture medium with 5% FBS, 1% endothelial cell growth factor, 100 U/mL penicillin, and 100 U/mL streptomycin. Extracts were prepared following ISO 10993-12-2021: autoclaved materials were soaked in ECM medium at 200 mg/mL for 72 h at 37°C, filtered through a 0.22 µm membrane, and stored at 4°C.
HUVECs were pretreated with material extracts for 24 h and then seeded on growth factor-depleted Matrigel (BD Bioscience) in 48-well plates. Tube formation assays were conducted using optical microscopy (Olympus) at 2, 4, 8, and 12 h. At 2 h, cells had not yet formed tubular structures, whereas at 12 h, tubular structures began to break down (Figure S1). Subsequently, the images of 4 and 8 h were chosen for quantitative analysis using Image J software.
HUVECs (1×105 cells/well) were seeded in 12-well plates and cultured in extracts with serum until 100% confluency. Linear scratches were made, rinsed with PBS, and images were captured at 0 and 12 h postcultivation in serum-free extracts to assess wound healing via optical microscopy (Olympus) and Image J software.
For osteogenic induction, hJBMMSCs were treated with an osteogenic medium containing 50 µg/mL ascorbic acid (Sigma), 2 mM β-glycerophosphate (Sigma), and 100 nM dexamethasone (Sigma) until the cell confluence reached 70–80%.
hJBMMSCs were cultured with material samples for 7 days under osteogenic induction, fixed with 4% PFA, and stained with BCIP/NBT solution (Beyotime) to assess alkaline phosphatase (ALP) activity. For alizarin red S (ARS) staining, cells were fixed after 21 days, stained with ARS (Cyagen), destained with 10% cetylpyridinium chloride (Sigma), and the calcium concentration was measured using a spectrophotometer (BioTek) at 562 nm.
After fixation with 4% PFA and permeabilization with 0.3% Triton X-100, cells were blocked with 5% BSA (Solarbio). HUVECs were immunostained at day 3 with anti-VEGF primary antibody (1:200, Abclonal) overnight at 4°C, followed by TRITC fluorescent secondary antibody (1:400, Bioss) for 1 h at room temperature; hJBMMSCs at 7 days postosteogenic induction were stained with anti-BGLAP primary antibody (1:200, Abclonal) overnight at 4°C, followed by FITC fluorescent secondary antibody (1:400, Bioss) for 1 h at room temperature.
HUVECs were nurtured for 3 days and hJBMMSCs after 7 days osteogenic induction were collected for qRT-PCR. Total RNA was extracted from the samples using Trizol (Invitrogen) and cDNA was synthesized from 1 µg of total RNA using reverse transcriptase (Superscript II Preamplification System; Invitrogen). All the reactions were performed in triplicate and were normalized to the reference gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH). The specific primer sets used for this analysis are listed in Table S2.
2.5 In vivo measurements
The animal experiments conducted in this study received approval from Peking University's Ethics Review Board (PUIRB-LA2023268). All methods are reported in accordance with ARRIVE guidelines for animal experiments.27
Male Sprague-Dawley rats (8 weeks old, 200–250 g, six rats in each group, Charles River) were randomly assigned to Ti and Zr-BMG groups. Under pentobarbital-induced anesthesia, a medial parapatellar arthrotomy was performed to create a bone tunnel in the intercondylar fossa of the right femur, where a 12 mm long, 1.5 mm diameter cylindrical implant was inserted (Figure S2). After 8 weeks, rats were euthanized, blood was collected for biochemical tests, and femurs with implants along with liver, kidney, and spleen were fixed in 4% PFA for further processing.
Femurs with implanted samples underwent graded dehydration, embedding in light-curing epoxy resin, and preparation of thin sections using a grinding machine (Exakt). These sections were stained with Stevenel's blue and Van Gieson's picrofuchsin stain, followed by analysis using a light microscope (Leica).
Blood sample was then drawn from each rat and subjected to analysis using a Clinical Analyzer (BK-280, Biobase). Liver, kidney, and spleen tissues from rats underwent H&E staining after deparaffinization and rehydration. The sections were examined using a light microscope (BX53, Olympus).
2.6 Statistical analysis
The analysis of statistical data was executed using Graph Prism 8.0.1. The presentation of data encompassed mean values along with their corresponding standard deviations (SD). The Shapiro−Wilk test was employed to verify the normal distribution of the original dataset, and for the evaluation of significance relied on both the two-tailed independent Student's t test. Differences were considered statistically significant at: * for p < .05, ** for p < .01, and *** for p < .001.
3 RESULTS
3.1 Structural properties, thermal parameters, and surface chemical compositions
The new amorphous alloy, Zr61Cu23Al12Ti2Ag2 (at.%) (Zr-BMG), demonstrated its critical molding dimensions in Supplementary Materials (Figure S3): a 5 mm diameter cylinder (i) and 16 mm wide rectangles (ii). Figure 1A displays the DSC curve of the novel Zr-BMG, indicating a glass transition temperature (Tg) of 662.01 K and a crystallization temperature (Tx) of 720.30 K, resulting in a supercooled liquid range (∆T) of 58.29 K. The results demonstrated the system's superior GFA and thermal stability.9
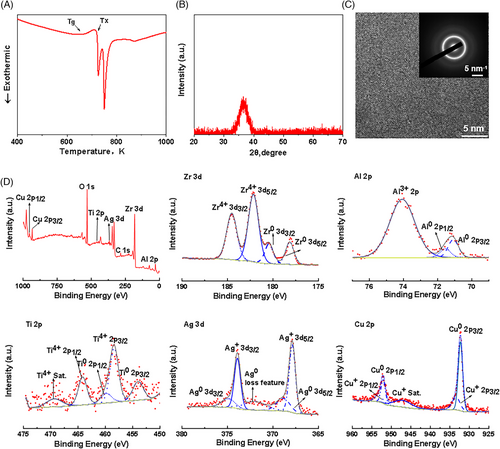
The XRD diagram shown in Figure 1B displayed a characteristic wide scattering peak, indicating the amorphous nature of the Zr-BMG material. Microstructural homogeneity was carefully scrutinized by TEM and two diffuse halos featuring the short-range order of BMGs confirmed the amorphous state of the alloys (Figure 1C).
SEM and EDS identified the presence of Zr, Cu, Al, Ti, Ag, and O in BMGs (Figure S4), confirming our elemental composition. XPS image (Figure 1D) and fine peak analysis of zirconium elements revealed that zirconium in the sample primarily existed in the Zr4+ state (182.15/184.53 eV), indicating that the tetravalent zirconium originates from zirconium oxide. Similarly, aluminum, titanium, and silver were also enriched in oxide forms, detected as Al3+, Ti4+, and Ag+, respectively. In contrast, Cu mainly existed in the zero-valent form. Detailed information of XPS peak analysis can be found in Supplementary Materials (Table S1). Based on the XPS results, it could be inferred that the predominant Zr element in Zr-BMG primarily existed on the material surface in the form of oxides, indicating the formation of an oxide layer on its surface. Additionally, Cu primarily existed on the material surface in the form of metallic Cu0.
3.2 The mechanical properties, hydrophilic characterization, and corrosion resistance
Compressive stress-strain curves and load-displacement curves of all samples are shown in Figure 2A,B. The compressive strength of Zr-BMG (1603.67±98.80 MPa) was about three times that of Ti (550.33±37.00 MPa) (Table 1). The reduced Young's modulus of Zr-BMG was 94.20±7.16 GPa (Table 1), which was closer to the reported cortical bone modulus (30 GPa) than Ti. Meanwhile, the microhardness of Zr-BMG was 5.30 GPa, which was greater than that of Ti (4.02 GPa). The mass density of the Zr-BMG, measured by the Archimedean technique with an accuracy of 0.1%, was approximately 6.27 g/cm3 (Table 1). Calculations revealed that the Zr-BMG exhibited a specific strength of 237.64 MPa cm3/g, which was higher than that of Ti (115.13 MPa cm3/g).
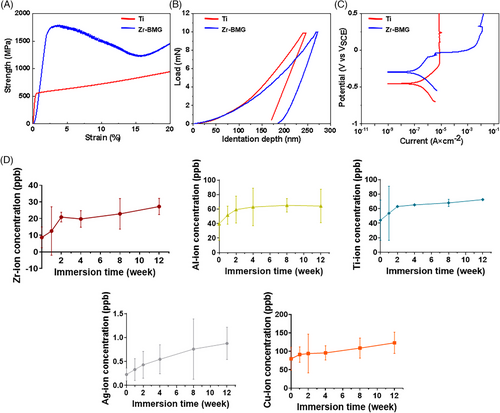
Figure 2C displays the polarization curves of Ti and Zr-BMG, the pitting corrosion potential of Zr-BMG was −0.06V versus saturated calomel electrode (VSCE), the curve also clearly illustrated the formation of a passive region for Zr-BMG, with a width of approximately 0.03V versus VSCE, indicating the development of a passive film on the material's surface. Table 2 summarizes essential electrochemical parameters obtained from the potentiodynamic polarization curves, Zr-BMG exhibited a higher self-corrosion potential and lower self-corrosion current density compared to Ti. Consequently, in the SBF environment, Zr-BMG demonstrated superior corrosion resistance to Ti, with the formation of a protective passive film on its surface. Considering the XPS results, the main component of the formed passive film was ZrO2.
The cumulative ionic concentrations released by the Zr-BMG in the SBF solution over time are depicted in Figure 2D. During the initial 2 weeks, the concentrations of all metal ions released increased rapidly with prolonged immersion time. However, from weeks 2 to 12, the rate of increase in metal ion release concentrations notably decreased, gradually reaching a stable level. This slow and sustained metal ion release from Zr-BMG avoided the adverse effects associated with rapid increases in metal ion concentrations over a short period.
The surface contact angle results with SBF for the two materials showed no significant differences (Figure S5).
3.3 In vitro measurements
3.3.1 Biocompatibility
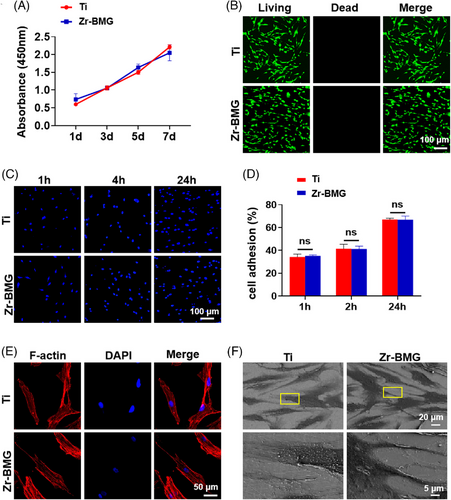
Cell adhesion and spreading on biomaterial surfaces are crucial, as cells are highly sensitive to the physical and chemical properties of materials. Figure 3A demonstrates the CCK-8 assay results, revealing similar cell proliferation rates on Zr-BMG and Ti surfaces at intervals of 1, 3, 5, and 7 days. Figure 3B presents the results of cell viability of hJBMMSCs culturing on Zr-BMG or Ti for 24 h, indicating no acute cytotoxicity. This suggested that both materials had a comparable effect on the proliferation of hJBMMSCs in this experimental context. As depicted in Figure 3C, hJBMMSCs were evenly distributed on the surfaces of all groups. Statistical analysis confirmed no significant difference between the two groups (Figure 3D). Cellular cytoskeleton staining (Figure 3E) revealed similar morphologies of cells adhered to both Zr-BMG and Ti surfaces. SEM images (Figure 3F) demonstrated comparable attachment and growth morphologies of cells on both materials.
3.3.2 Antibacterial properties assessment
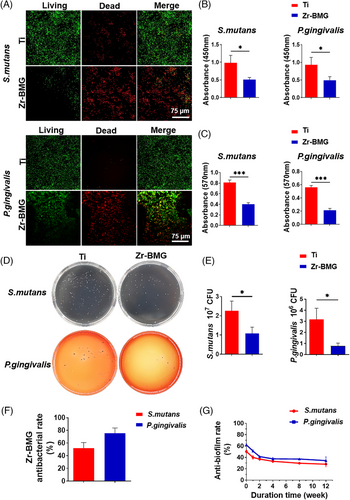
Implant-related infections are common postimplantation complications, often due to bacterial adhesion and subsequent biofilm formation on material surfaces.19 In this study, we selected two common oral pathogens, P. gingivalis and S. mutans, to evaluate the antibacterial performance of the developed biomaterials after coculturing with the materials. Figure 4A displays live/dead staining of bacteria on Ti and Zr-BMG samples. In contrast to the widespread presence of living bacteria on the Ti surface, less bacteria were found adhered to the Zr-BMG surface, among which largely were dead colonies. The result of the XTT assay demonstrated the bacterial proliferation on the Zr-BMG surface was significantly inhibited, indicating its superior ability to suppress the bacterial activity of P. gingivalis and S. mutans (Figure 4B). Figure 4C demonstrates that bacterial biofilms on the Zr-BMG surface were significantly inhibited. To further assess the antibacterial effect of Zr-BMG on S. mutans and P. gingivalis, adhered bacteria were ultrasonically removed from the material surface and counted for colony formation (Figure 4D). The results depicted in Figure 4E demonstrated a significant decrease in colony numbers for the Zr-BMG group, with approximately 52% of S. mutans and 76% of P. gingivalis strains being eliminated compared to the Ti control, as shown in Figure 4F. This indicated the superior antibacterial effectiveness of the Zr-BMG material. Using crystal violet staining for quantification, we assessed the long-term antibacterial biofilm formation on Zr-BMG surfaces. Figure 4G illustrates an initial rapid decline in Zr-BMG's biofilm inhibition efficacy, stabilizing from weeks 2 to 4, and maintaining consistent bactericidal activity. Even after 3 months, Zr-BMG retained a biofilm inhibition rate of 36.42% for S. mutans and 44.00% for P. gingivalis.
3.3.3 Antibacterial mechanism
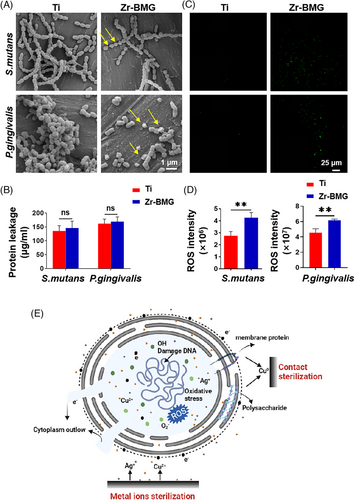
This study utilized SEM to observe bacterial morphology and distribution on materials. Figure 5A shows bacteria inoculated on Ti exhibited spherical shapes with smooth surfaces. In contrast, those on Zr-BMG displayed extensive cell wall ruptures and membrane disruptions, with observable twisted and completely detached membrane structures. Concurrently, this study measured the bacterial protein release on different materials. Figure 5B illustrates that bacteria inoculated on Zr-BMG exhibited a slightly higher protein release compared to those on other materials, yet the difference was not statistically significant (p > .05). Reactive oxygen species (ROS) are integral to cellular physiological processes, with excessive ROS leading to oxidative stress and cell death.28, 29 As depicted in Figure 5C, higher ROS production was observed on bacteria adhered on the Zr-BMG surface. In the meantime, significant ROS signals were detected in the bacterial suspension in the Zr-BMG group (Figure 5D). Therefore, we speculated that the antibacterial mechanism of this material might include both contact sterilization and ion sterilization (Figure 5E). When bacteria came into contact with the material, the Cu on the surface could kill the bacteria by disrupting structures such as the bacterial cell membrane. Simultaneously, the material could release copper and silver ions in the SBF solution, inducing substantial ROS production in bacteria, elevating intracellular ROS levels, causing oxidative stress damage, and leading to bacterial cell death.
3.3.4 Angiogenesis and osteogenesis ability
Angiogenesis is a critical process during bone integration, where new blood vessels provide oxygen and nutrients to surrounding cells to promote their proliferation and differentiation. HUVECs cultured in material extracts underwent scratch assays (Figure 6A), and quantitative analysis (Figure S6) demonstrated that Zr-BMG outperformed Ti in promoting wound healing. Moreover, we closely examined tube formation in HUVECs at 4 - and 8-h intervals through microscopic observation. Figure 6B shows that Zr-BMG led to denser and more widely distributed microvascular networks with fewer isolated cells compared to Ti. Statistical analysis in Figure 6C revealed that Zr-BMG significantly enhanced angiogenesis, as evidenced by total tubular length (p < .01) and network count (p < .05). qRT-PCR analysis demonstrated a significant upregulation of angiogenesis-related markers, including VEGF, ANG2, and APLN, in Zr-BMG after 3 days of coculturing (Figure 6D). Immunofluorescent staining further confirmed the elevated protein expression of VEGF in Zr-BMG compared to Ti (Figure 6E).

This study explored the osteogenesis capabilities of Zr-BMG by employing a jawbone-specific in vitro model, utilizing hJBMMSCs, thereby capturing the unique osteogenic feature of the jawbone30 to evaluate the potential of Zr-BMG as a dental implant material. hJBMMSCs were directly cultured on materials’ surface to evaluate their osteogenic potential. ALP is an early marker of osteogenic differentiation in hJBMMSCs.31 Mineralized nodules are considered as indicators of mature and late-stage osteogenesis.31 Figure 6F shows a significant increase in ALP activity in hJBMMSCs on Zr-BMG compared to Ti, as revealed by statistical analysis (p < .001) (Figure 6H). Subsequently, the same cell types were induced for osteogenic differentiation for a period of 14 days and then stained with ARS. The results, displayed in Figure 6G, showed that cells on Zr-BMG developed considerably larger calcium nodules with more intense staining than those on the Ti substrate. A further quantitative analysis (Figure 6H) of the staining confirmed that Zr-BMG significantly enhanced the extracellular matrix mineralization capabilities of hJBMMSCs postosteogenic differentiation, demonstrating its superior osteoinductive potential. After 3 days of osteogenic induction of cells on Ti and Zr-BMG, the expression levels of bone-related mRNAs were presented in Figure 6I. In this study, we assessed the expression of osteogenesis-associated factors, RUNX2, COL-1A, BGLAP, and SPP1. Our analysis revealed a significant upregulation of these factors in cells cultured on the surface of Zr-BMG, with statistically significant differences between the two groups (p < .05). Immunofluorescence analysis in Figure 6J revealed that, when compared to Ti, the expression of BGLAP in hJBMMSCs cultured on Zr-BMG was higher.
3.4 In vivo measurements
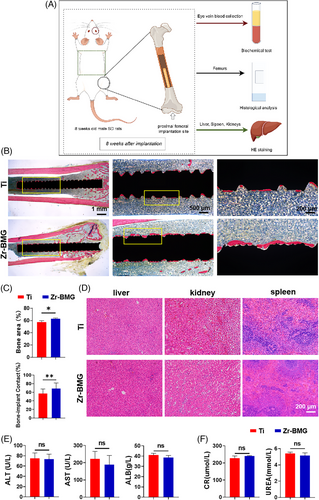
Establishing direct contact between bone and implant without the presence of fibrous tissue is crucial for successful osseointegration.32 To further analyze the osteogenic effects of Zr-BMG on cancellous bone, an alcian blue-acid fuchsin stain was performed to assess the osseointegration of Ti and Zr-BMG implants after 8 weeks (Figure 7A). As shown in Figure 7B, osteoid tissue appeared blue-green and newly mineralized bone appeared deep red. More bone-like tissues were formed in direct contact with the Zr-BMG, in comparison with Ti control at 8 weeks postimplantation surgery. Histological analysis of hard tissue sections supported a significantly larger bone-implant contact area (p < .05) and length (p < .01) around Zr-BMG implants than Ti at 8 weeks postsurgery (Figure 7C), indicating a superior osseointegration ability of Zr-BMG compared to Ti. Although Zr-BMG contained complex metallic elements, researchers had not observed significant pathological changes in the liver, kidneys, or spleens (Figure 7D). Similarly, no signs of pathological features were observed in serum tests of rats implanted with Ti and Zr-BMG after 8 weeks (Figure 7E,F).
4 DISCUSSION
The addition of Ag enhanced the alloy's GFA, meeting the criteria outlined by Inoue,9 which included three or more elements, atomic size mismatch exceeding 12%, and negative mixing enthalpy between constituent elements. In the Zr-Ti-Cu-Al-Ag system, Ag exhibited negative mixing enthalpies with Zr, Ti, and Al (Table S2),33 fostering chemical short-range ordering in the liquid state, enhancing local packing efficiency, and suppressing long-range atomic diffusion for improved GFA. From an atomic scale perspective, Ag was incorporated into the Zr-Ti-Cu-Al alloy due to its chemical similarity to Cu but with a different atomic size (RAg/Cu = 0.810), which was expected to enhance the dense atomic packing in the glass, thereby promoting GFA.34
The compressive strength of the Zr-BMG was 1603.67 MPa, with an elastic modulus of 94.20 GPa, consistent with most reported Zr-Cu-Al metallic glasses (strength ranging from 1600 to 2000 MPa and modulus from 80 to 100 GPa).12, 35 Studies had indicated a linear relationship between strength and elastic modulus.12, 36 Clinically used implant materials often could not achieve both a relatively low elastic modulus and high strength simultaneously. Table 3 compares the strength and elastic modulus of these materials to that of cortical bone. The Zr-BMG exhibited balanced strength and elastic modulus, making it suitable for implants. Meanwhile, the Zr-BMG demonstrated elevated microhardness compared to Ti, implying enhanced resistance to wear and a possible decrease in osteolysis triggered by wear debris, commonly referred to as particle-induced disease.37 Additionally, Zr-BMG exhibited higher specific strength than Ti, thus allowing it to achieve the same strength with a smaller cross-sectional area as Ti,38 thereby reducing the diameter of the implant itself.
| Materials | Density (g/cm3) | Compressive strength (MPa) | Reduced Young's modulus (GPa) | Microhardness (GPa) | Contact angle (°) |
|---|---|---|---|---|---|
| Ti | 4.78 ± 0.02 | 550.33 ± 37.00 | 120.45 ± 17.13 | 4.02 ± 0.91 | 53.63 ± 2.17 |
| Zr-BMG | 6.27 ± 0.01 | 1603.67 ± 98.80 | 94.20 ± 7.16 | 5.30 ± 1.27 | 54.14 ± 4.61 |
- Note: Data were presented as mean ± SD.
| Materials | Ecorr (V VS SCE) | Icorr (A/cm2) | Passive region (V vs. SCE) | Pitting corrosion potential (V vs. SCE) |
|---|---|---|---|---|
| Ti | −0.45 | 8.53 × 10−8 | − | − |
| Zr-BMG | −0.30 | 1.09 × 10−7 | 0.03 | −0.06 |
In general, the corrosion resistance is determined by the nature and characteristics of the passivation film on the alloy surface.40 Analysis of the XPS results revealed that the excellent corrosion resistance of Zr-BMG was attributed to the formation of a highly protective surface film enriched with Zr4+, which was consistent with the findings of Hua et al.41
Bone integration and vascularization are closely intertwined processes, crucial for successful implantation.42 ICP analysis showed that Zr-BMG could release Cu2+ in SBF, and its concentration could reach 98.70 pbb. It had been reported that Cu was a key element that affected blood vessel growth and could stimulate blood vessel production at the molecular level.23 Li et al. demonstrated that the Zr-Ti-Cu-Al BMG significantly enhanced the expression of VEGF, thus contributing to the regulation of vasculogenesis.24 Another study found that HUVECs cultured on Zr-Ti-Cu-Al BMG showed higher proliferation, evidenced by significantly higher OD values starting from day 7, and enhanced differentiation with higher gene expression levels of VEGF compared to those cultured on Ti at day 7. These findings aligned with our in vitro experiments using HUVECs.43 Therefore, we reasonably speculated that the Cu2+ had contributed to promote angiogenesis.
In our study, we not only confirmed the bactericidal efficacy of Zr-BMG against two common oral pathogens, P. gingivalis and S. mutans, but also demonstrated its capability to effectively disrupt bacterial biofilm formation over the long term. We conducted preliminary explorations into the mechanisms underlying its antibacterial properties. Two main views on the antibacterial mechanism of Cu- and Ag-containing alloys exist: (1) Contact sterilization, Cu particles contact the cell membrane, triggering a short-circuit effect, charge transfer, and proton depletion that ruptures the membrane. Microbial byproducts like NH4+ and CN− react with Ag, forming complexes that promote Ag dissolution and interact with the bacterial membrane, causing “contact sterilization.” (2) Metal ion sterilization. Cu ions generate ROS that damage membranes and proteins, contributing to bactericidal effects. They also enter bacterial cells, disrupting proteins, DNA replication, and RNA transcription. Ag ions dissolve in the microenvironment, interacting with bacteria similarly to Cu ions. After bacterial death, Ag ions bind to biomolecules, then are released to target other microorganisms, ensuring persistent antibacterial activity.21, 28, 29, 44-46 As shown in Figure 6E, we observed the morphology of bacteria in direct contact with the material using SEM. After 24 h of contact, bacteria on the surface of Zr-BMG displayed extensive cell wall ruptures and membrane disruptions, with observable twisted and completely detached membrane structures. Moreover, significant ROS signals were detected in the bacterial suspension in the Zr-BMG group. This might be related to the metal ions released by Zr-BMG. The dissolution of Ag ions led to the production of ROS, thereby influencing antibacterial properties. Similarly, the dissolution of Cu ions resulted in the loss of cell membrane potential and cytoplasm release, leading to cell membrane rupture and Cu ion-induced ROS generation, ultimately damaging bacterial structure. Additionally, ICP-MS experiments demonstrated that the release of Cu ions and Ag ions from Zr-BMG in SBF was stable, persisting for at least 12 weeks, significantly longer than the previously reported 42 days.46 This indicated that Zr-BMG could exert a sustained and stable antibacterial effect. The osseointegration period for dental implants is typically 3 months. Therefore, this study selected a 3-month timeframe as the research endpoint. For long-term antibacterial evaluation, subsequent research will employ standardized duration tests (up to 12 months) with periodic metabolic activity assessments.
The present study introduced a novel alloy, Zr61Cu23Al12Ti2Ag2 (at.%), which had promising potential for clinical application as a dental implant material. However, this study had several limitations. First, the lack of ductility in Zr-BMG severely limited its machinability using conventional processing methods. In the future, we will explore methods for processing metallic glasses such as additive manufacturing. Second, there was still a lack of material science mechanism studies regarding the advantages of the developed Zr-BMG. Future studies would include control groups of Zr-based BMG with different elemental compositions to explore these mechanisms, potentially guiding the development of future materials. Third, we were unable to confirm the intracellular signaling of this study result. To verify the biological mechanism, experiments such as next-generation sequencing or proteomics analysis were required. Lastly, the impressive mechanical properties and antibacterial effects of Zr-BMG highlight its potential for use in fracture fixation devices. Its high strength and optimal elastic modulus make it well-suited for load-bearing applications, while its antimicrobial properties could help reduce the risk of infections. However, further research is required to evaluate its long-term performance and effectiveness in orthopedic settings, particularly in terms of its durability and biocompatibility under physiological conditions.
5 CONCLUSIONS
This study presented a novel amorphous alloy, Zr61Cu23Al12Ti2Ag2 (at.%) (Zr-BMG), as a promising alternative to traditional Ti alloys for dental implants. Zr-BMG exhibited a critical size of up to 5 mm, meeting the manufacturing requirements for dental implants fabrication. Its compressive strength was approximately three times greater than that of Ti, while its elastic modulus was only two-thirds of Ti's. These properties aligned more closely with the biomechanical needs of dental implant materials. Furthermore, Zr-BMG also demonstrated significant antibacterial properties, effectively combating two common oral pathogens, S. mutans and P. gingivalis. Notably, its antibacterial effect lasted for up to 3 months, which potentially reduced the risk of peri-implantitis, a common complication associated with dental implants. This extended antimicrobial activity is a key advantage over traditional materials, which typically offer limited protection. In addition to its mechanical and antimicrobial properties, Zr-BMG exhibited excellent angiogenic and osteogenic capabilities. These features were expected to promote faster and more efficient osseointegration, potentially shortening the integration period of dental implants. Given these advantages, Zr-BMG represented a highly promising material for improving the longevity and performance of dental implants, offering enhanced mechanical properties, sustained antibacterial protection, and improved biological compatibility.
AUTHOR CONTRIBUTIONS
Feifei Wang, Yunshu Wu and Fu Zheng contributed equally to this work as co-first authors. The authors thank Yuhao Zeng for his kind assistance in in vivo implantation surgery, Zifan Zhao for his support in materials preparation, and Yao Huang for the acquisition of TEM images. Xu Zhang, Baoan Sun, and Yuchun Sun supervised this work, they are listed as co-corresponding authors.
ACKNOWLEDGMENTS
This project was supported by the Youth Fund of the National Natural Science Foundation of China (82201125), the National Natural Science Foundation of China (62488201, 52192602).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.