Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
RESEARCH ARTICLE
A New Route for the Synthesis of (Trichloromethyl)Quinazoline-4(1H )-One and (1,2,3-Triazole)-Quinazoline-4(1H )-One Functionalized Derivatives via Copper-Catalyzed One-Pot Multicomponent Reactions
- Pages: 1914-1923
- First Published: 27 September 2024
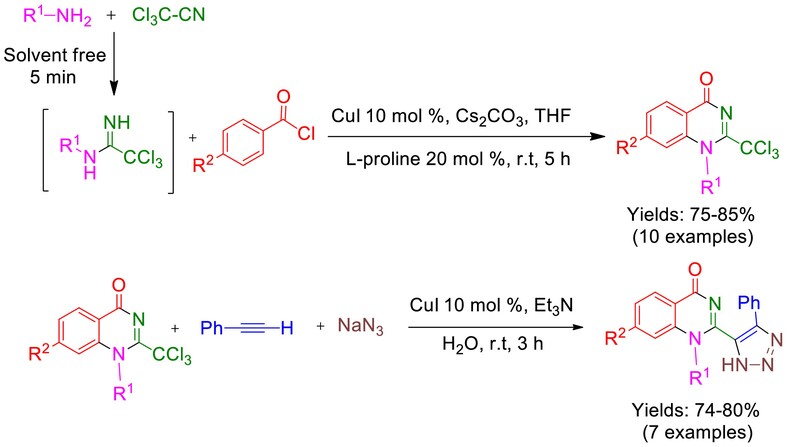
The synthesis of functionalized (trichloromethyl)quinazoline-4(1H)-one through a novel intramolecular CH activation reaction from trichloroacetonitrile, benzoyl chlorides, and various primary amines is a remarkable achievement in organic chemistry. Furthermore, the product with phenylacetylene and sodium azide in the presence of a copper catalyst in water solvent at room temperature demonstrates a new (1,2,3-triazole)-quinazoline-4(1H)-one derivative.
Benzenediazonium Tetrafluoroborate-Catalyzed Formal [3 + 3] Cyclization of 4-Hydroxycoumarins With Propargylicalcohols
- Pages: 1924-1931
- First Published: 27 September 2024
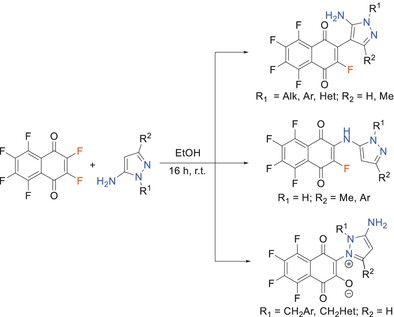
The Study of Reaction of Hexafluoro-1,4-Napthoquinone With Substituted 5-Aminopyrazoles
- Pages: 1932-1941
- First Published: 30 September 2024
Practical Copper-Catalyzed Double N-Arylation of Cyclic Diaryliodoniums: Synthesis of 5H-Dibenzo[d, f][1,3]Diazepine, and Benzo[c]Cinnoline Derivatives
- Pages: 1942-1953
- First Published: 30 September 2024
![Practical Copper-Catalyzed Double N-Arylation of Cyclic Diaryliodoniums: Synthesis of 5H-Dibenzo[d, f][1,3]Diazepine, and Benzo[c]Cinnoline Derivatives](/cms/asset/f551b59e-590e-4eab-856a-202d28cbf60b/jhet4914-toc-0001-m.jpg)
An efficient access to novel families of 7-membered dibenzodiazepines and 6-membered benzo[c]cinnoline derivatives has been elaborated. The synthetic strategy is based on a copper-catalyzed double N-arylation of cyclic diaryliodoniums with imidamides and 4-substituted 1, 2, 4-triazoline-3, 5-diones (TADs) respectively under ambient reaction conditions. A mechanistic rationale for the double N-arylation is discussed.
SHORT COMMUNICATION
GaCl3 -Catalyzed [3 + 2]-Cycloaddition/Esterification Cascade of Donor–Acceptor Cyclopropanes With Salicylaldehydes for the Synthesis of Tetrahydro-4 H-Furo[3,2-c]Chromen-4-Ones
- Pages: 1953-1965
- First Published: 30 September 2024
RESEARCH ARTICLE
Design, Synthesis, In Vitro Evaluation of New Tetrahydrooxazolo [5′,4′:4,5]Pyrimido[1,2-a]Azepinone Derivatives as Anticancer Agents
- Pages: 1966-1979
- First Published: 01 October 2024
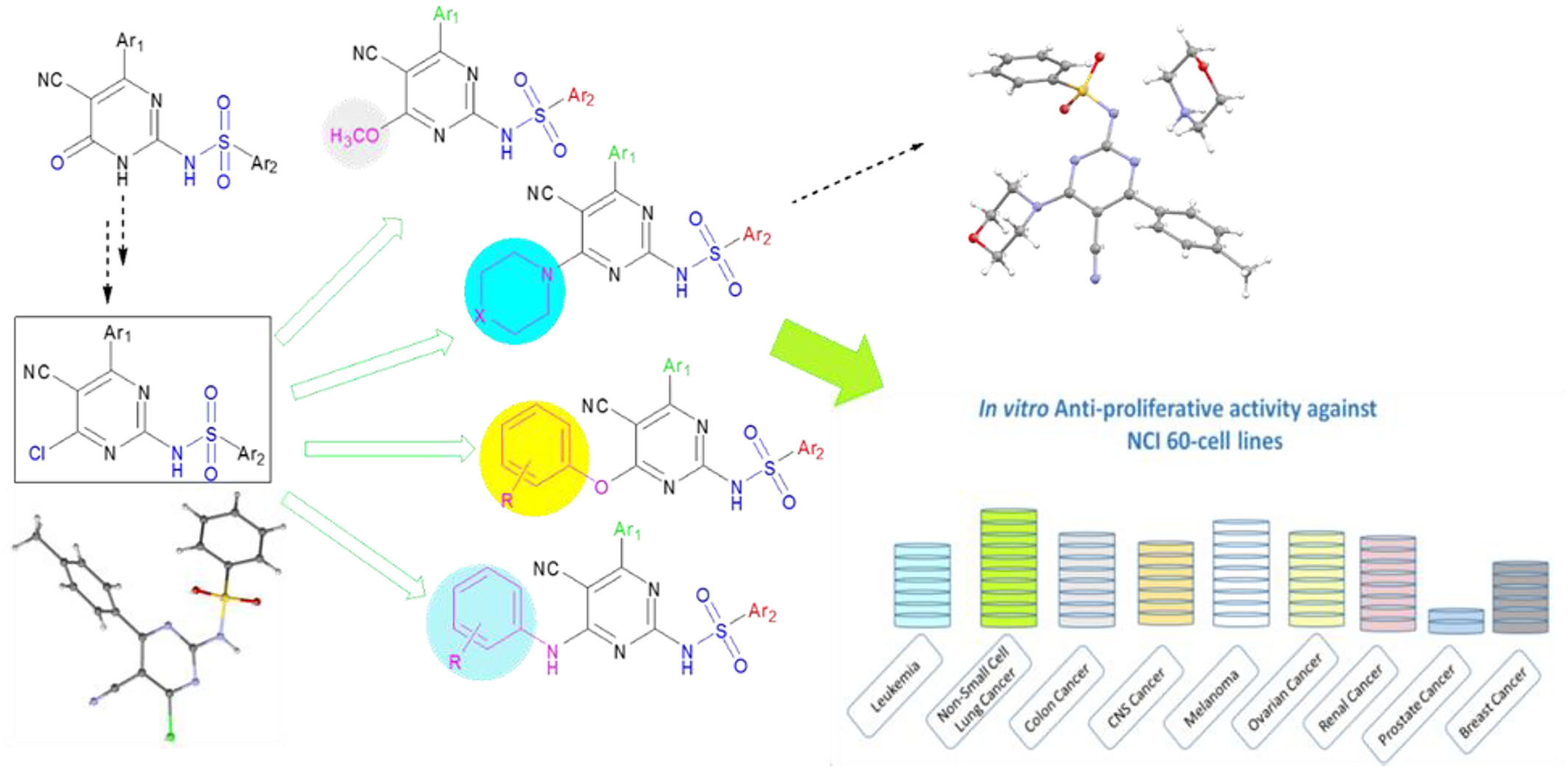
Discovery of Promising Sulfadiazine Derivatives With Anti-Proliferative Activity Against Tumor Cell Lines
- Pages: 1980-1998
- First Published: 02 October 2024
Synthesis of Benzannulated Spiroketals Derived From Stigmasterol and Sitosterol by Pd-Catalyzed Spirocyclization. NMR and X-ray Characterization. Evaluation of Cytotoxicity and Anti-Inflammatory Activity
- Pages: 1999-2014
- First Published: 02 October 2024
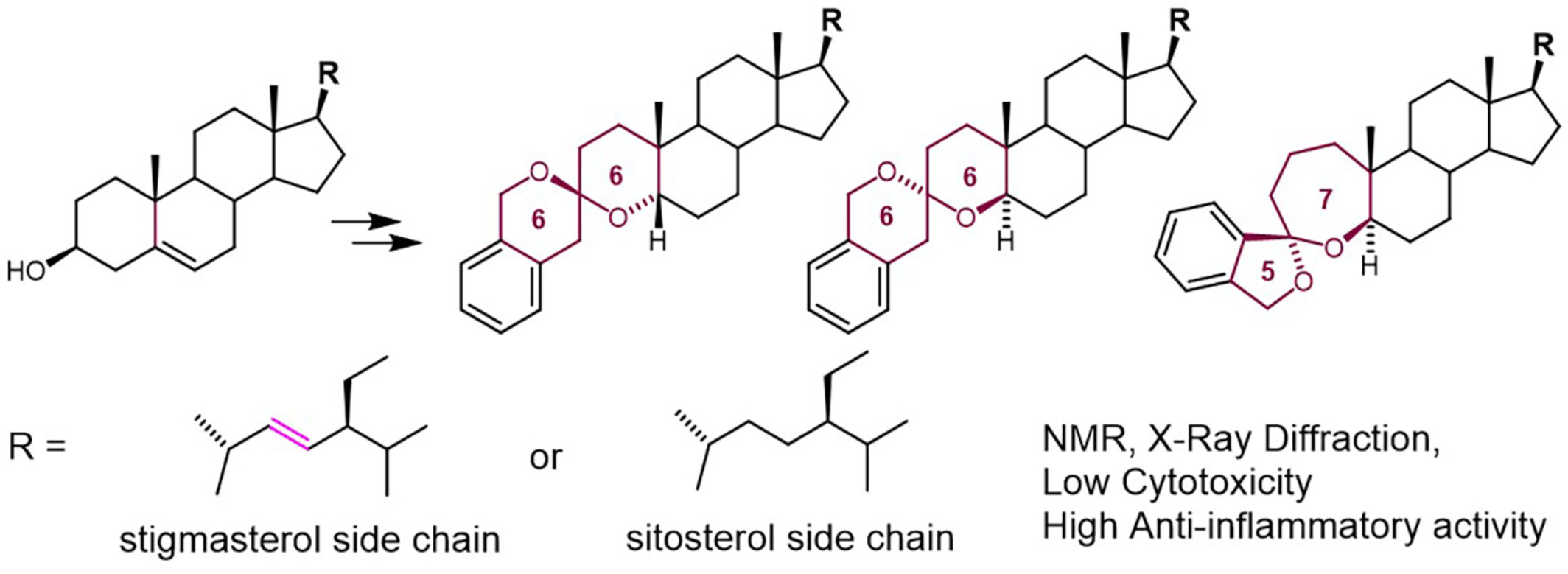
Five new benzannulated steroid spiroketals were synthesized by Pd-catalyzed spiroketalization of 5α and 5β-alkynediols derived from stigmasterol and sitosterol. The detailed NMR and X-ray characterization of the newly obtained spiroketals are presented. While the obtained compounds showed null or very low cytotoxicity, two of them inhibited more than 60% of the nitrous oxide production in J774A.1 macrophages stimulated with LPS, showing promising properties as anti-inflammatory agents.
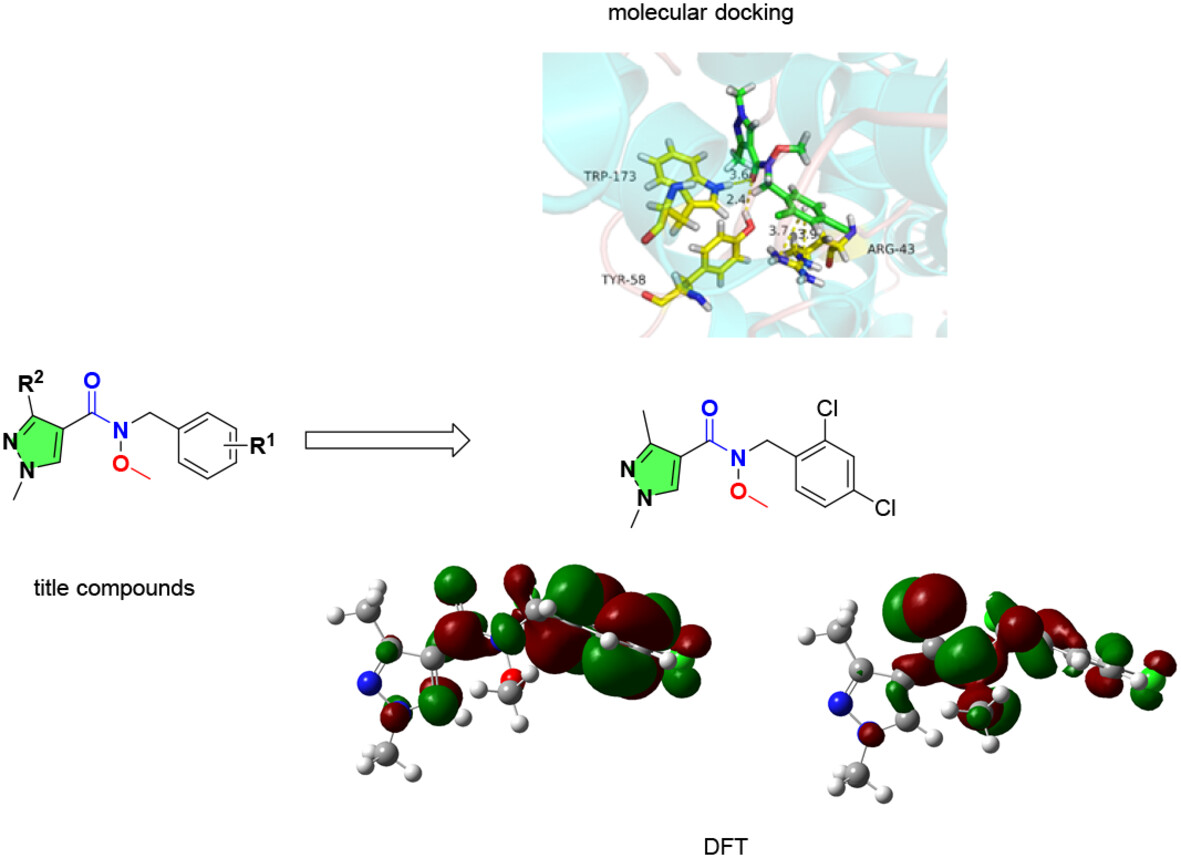
N-Methoxy Pyrazole-4-Carboxamide Derivatives: Synthesis, Spectral Analyses, Antifungal Activity, In Silico Molecular Docking, ADMET, and DFT Studies
- Pages: 2015-2025
- First Published: 02 October 2024
REVIEW
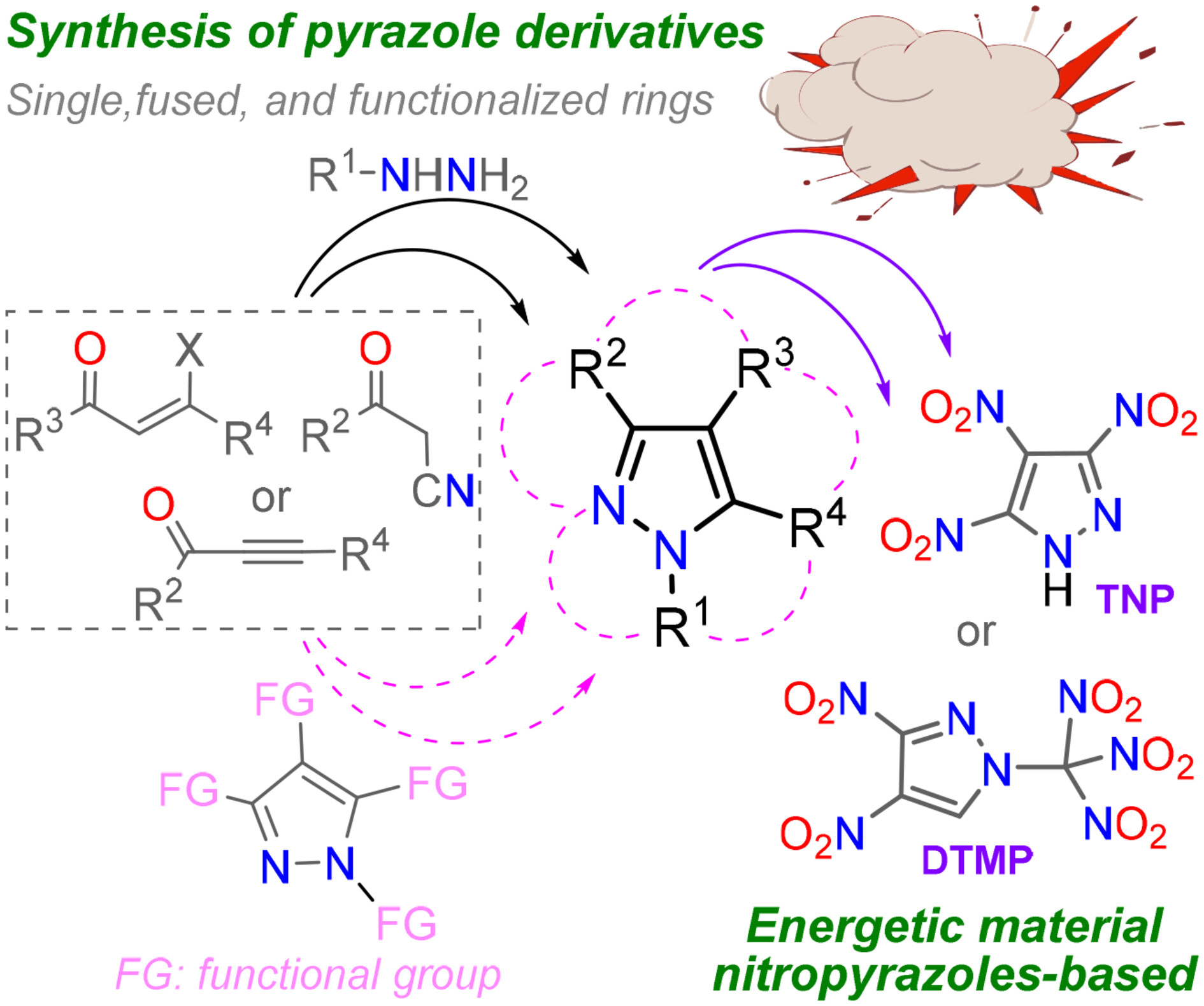
Current Advances in Synthesis of Pyrazole Derivatives: An Approach Toward Energetic Materials
- Pages: 2026-2039
- First Published: 02 October 2024
RESEARCH ARTICLE
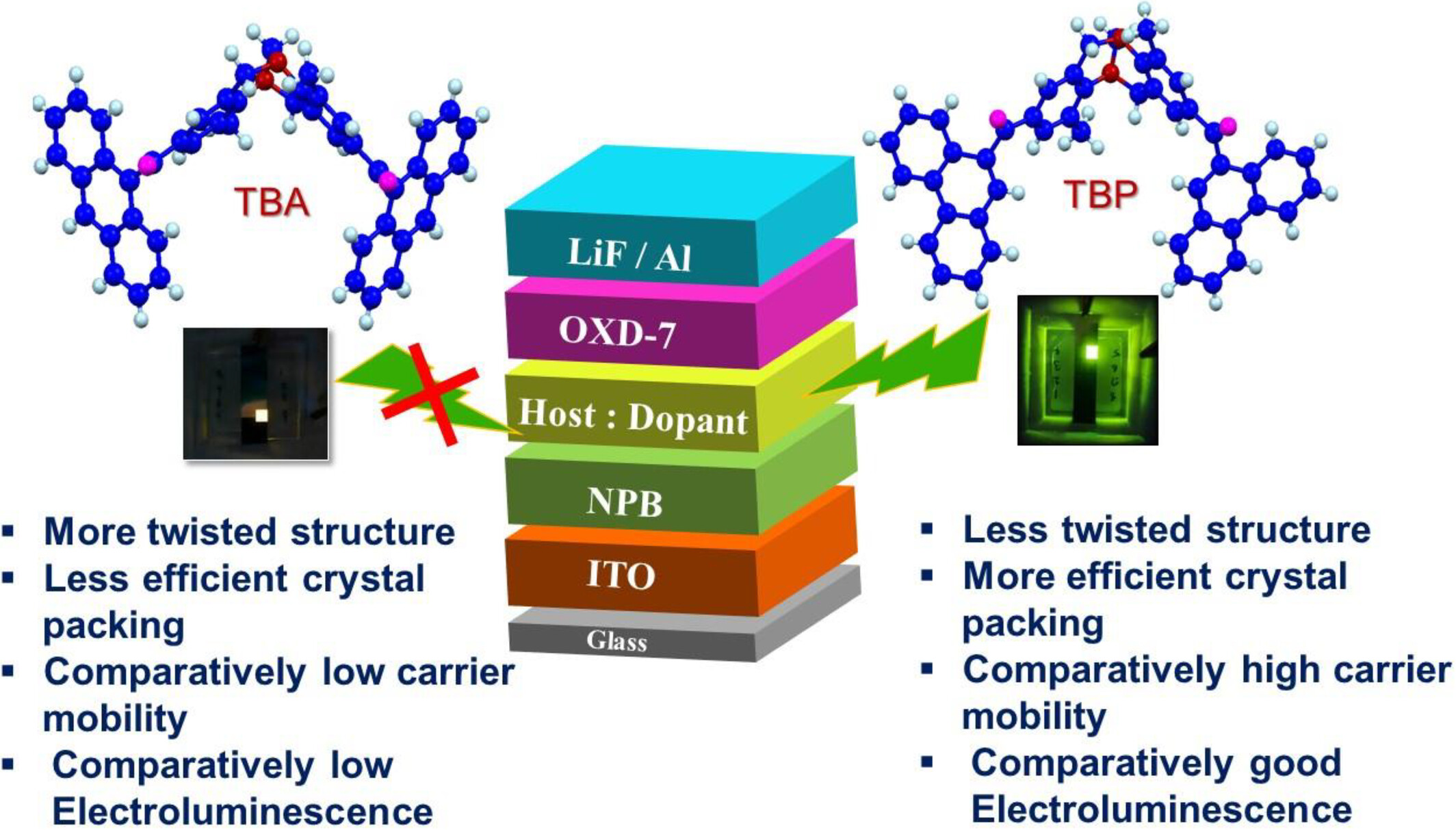
1,5-Diazocine-Based Diaryl Ketones: Design, Synthesis, and Optoelectronic Properties
- Pages: 2040-2049
- First Published: 04 October 2024
REVIEW
Visible Light Mediated CO2 Fixation Reactions to Produce Carbamates and Carbonates: A Comprehensive Review
- Pages: 2050-2069
- First Published: 08 October 2024
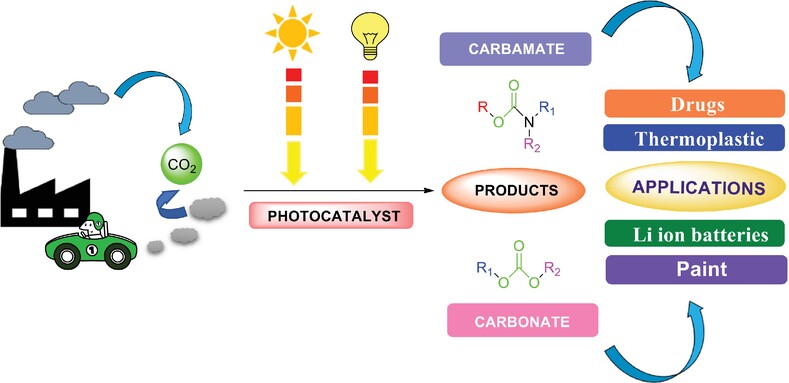
Visible light mediated synthesis of carbamates and carbonates through carbon dioxide (CO2) fixation using photocatalysts. Carbamates and carbonates are profitable and fascinating compounds for industrial applications where CO2 has been incorporated as a C1-synthon thus also serving in minimizing the harmful impact of CO2.
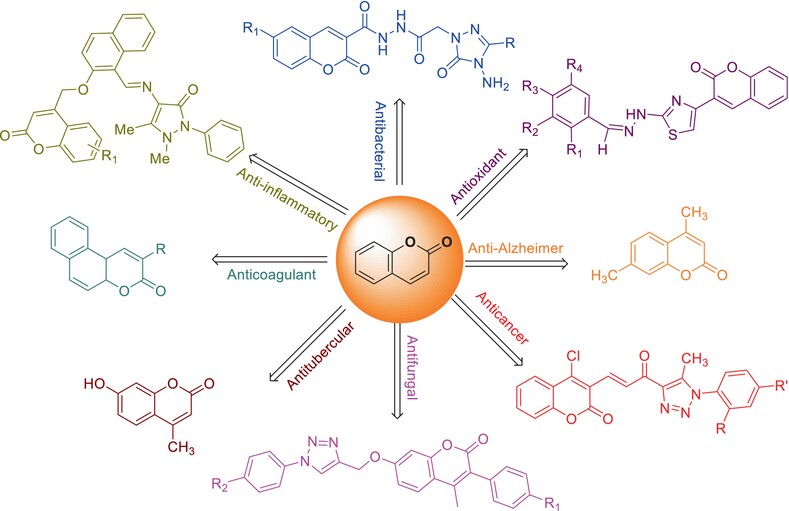
Coumarin Derivatives: Microwave Synthesis and Biological Properties—A Review
- Pages: 2070-2096
- First Published: 10 October 2024
RESEARCH ARTICLE
Lewis Base Promoted [4+2] Annulation of o-Acylamino-Aryl Morita-Baylis-Hillman Carbonates With Isatylidene Malononitriles: Facile Access to Spiro[Indolin-3,2′-Quinoline] Frameworks
- Pages: 2097-2105
- First Published: 10 October 2024
REVIEW
Synthetic Strategies for C-Amino 1,2,3-Triazoles and Their Oxides: A Review
- Pages: 2106-2125
- First Published: 12 October 2024





![Benzenediazonium Tetrafluoroborate-Catalyzed Formal [3 + 3] Cyclization of 4-Hydroxycoumarins With Propargylicalcohols](/cms/asset/fc4342ec-5e04-4cc7-8e3f-da78e630458f/jhet4912-toc-0001-m.jpg)
![GaCl3
-Catalyzed [3 + 2]-Cycloaddition/Esterification Cascade of Donor–Acceptor Cyclopropanes With Salicylaldehydes for the Synthesis of Tetrahydro-4
H-Furo[3,2-c]Chromen-4-Ones](/cms/asset/1bb0e875-9505-44d2-986d-5e746b185fef/jhet4917-toc-0001-m.jpg)
![Design, Synthesis, In Vitro Evaluation of New Tetrahydrooxazolo [5′,4′:4,5]Pyrimido[1,2-a]Azepinone Derivatives as Anticancer Agents](/cms/asset/da5a741a-503c-4739-83d5-0857fdcd38b0/jhet4907-toc-0001-m.jpg)
![Lewis Base Promoted [4+2] Annulation of o-Acylamino-Aryl Morita-Baylis-Hillman Carbonates With Isatylidene Malononitriles: Facile Access to Spiro[Indolin-3,2′-Quinoline] Frameworks](/cms/asset/5207c7c4-df6d-4c26-b355-01818d8934bd/jhet4919-toc-0001-m.jpg)