Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
RESEARCH ARTICLE
Chiral Post-Modification of UiO-66-NH2 on Gas Chromatography Chiral Separation
- First Published: 17 July 2025
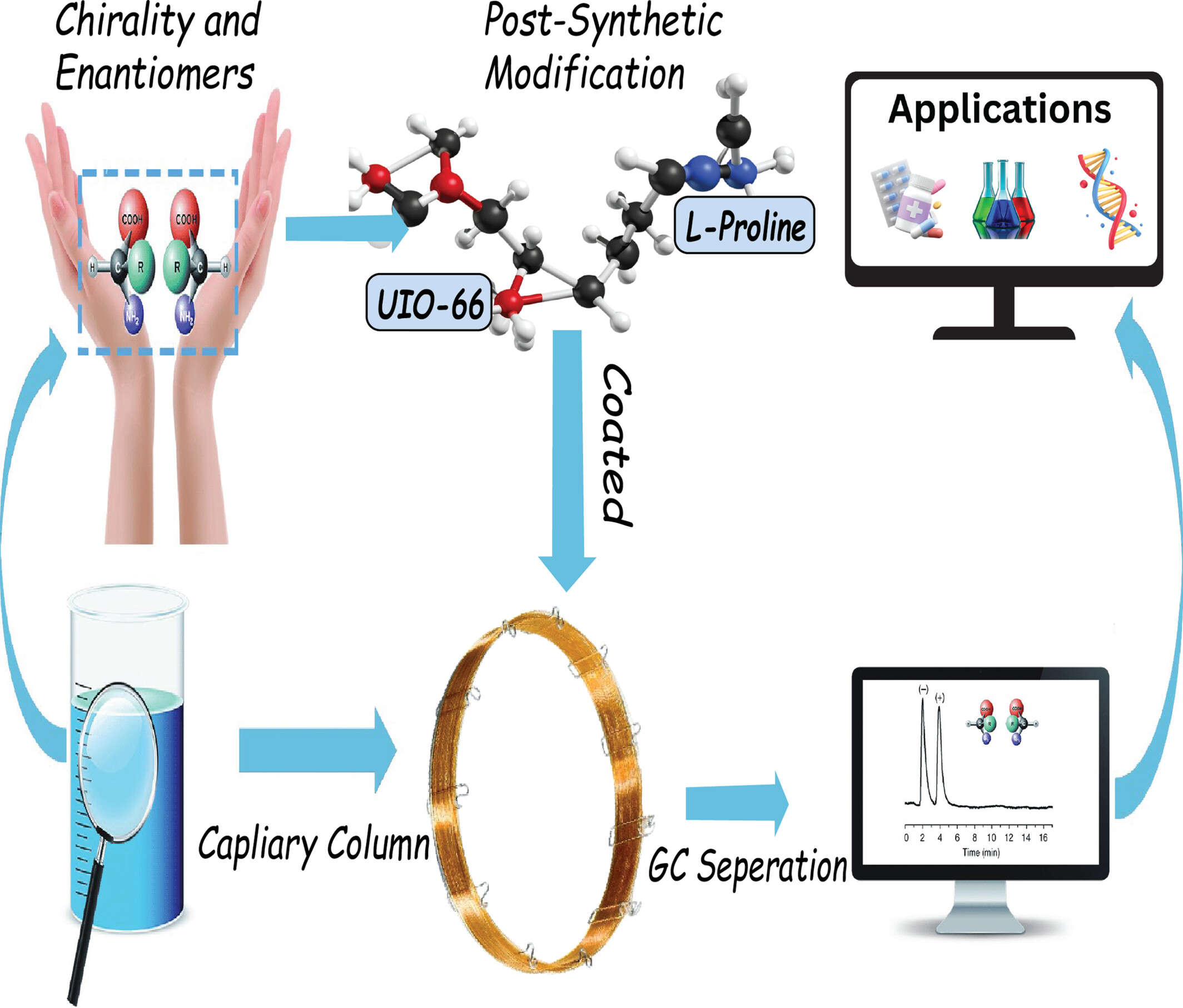
A particular type of zirconium metal–organic framework (MOF) called chiral UiO-66 was created to serve as a stationary phase in gas chromatography. It successfully achieved high-resolution separation of enantiomers, such as linalool and α-pinene, as well as positional isomers. This MOFs is effective for chiral analysis in the chemical and pharmaceutical industries.
Chiral Separation and Molecular Simulation of Five Quinolones on a Bonded Amylose[(S)-α-Methylbenzyl Carbamate] Column (CHIRALPAK IH) and the Elucidation of Its Recognition Mechanism
- First Published: 17 July 2025
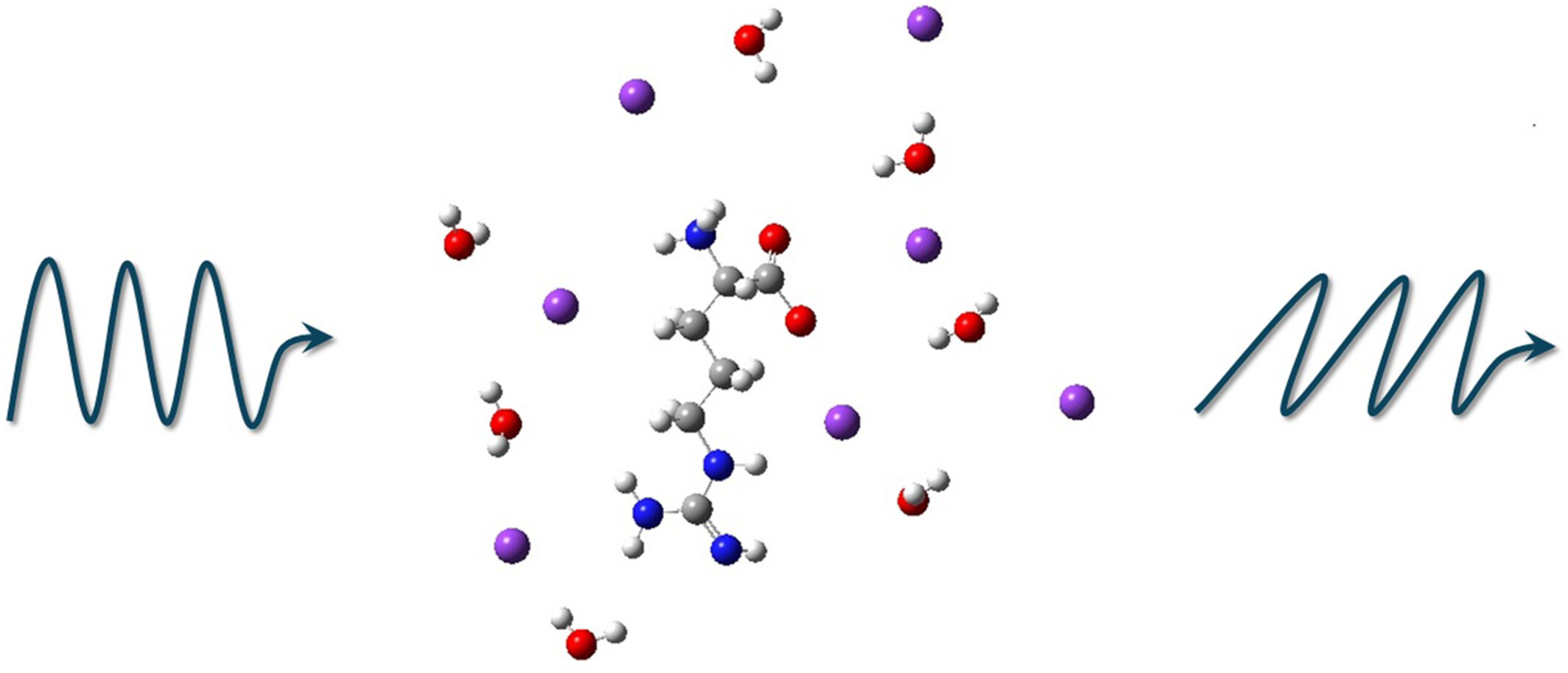
Variations in the Optical Activity of L-Arginine in Electrolyte and Nonelectrolyte Solutions
- First Published: 22 July 2025




![Chiral Separation and Molecular Simulation of Five Quinolones on a Bonded Amylose[(S)-α-Methylbenzyl Carbamate] Column (CHIRALPAK IH) and the Elucidation of Its Recognition Mechanism](/cms/asset/ed7f80c4-ff3f-4c4b-800c-05f17f11a6cb/chir70050-toc-0001-m.jpg)