Journal list menu
Export Citations
Download PDFs
COVER PICTURE
EDITORIAL BOARD
REVIEW ARTICLES
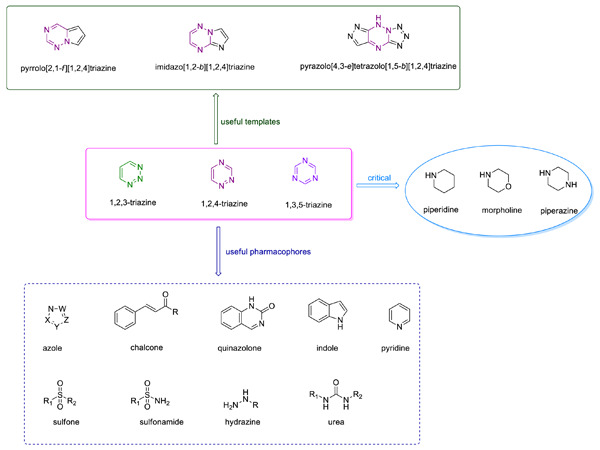
Recent updates on 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action
- First Published: 13 November 2022
Spotlight on 4-substituted quinolines as potential anti-infective agents: Journey beyond chloroquine
- First Published: 09 December 2022
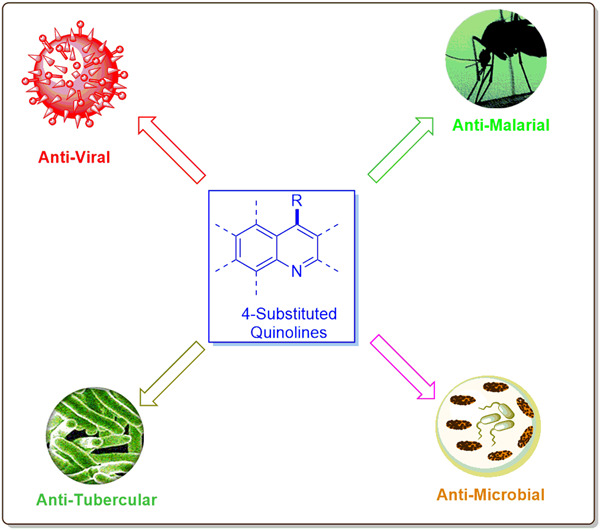
This review covers the biological potential of 4-substituted quinoline and gives a rationale for its special privilege to afford many approved clinical drugs, particularly against infectious pathogens. The anti-infection spectrum of these compounds against viruses, mycobacteria, malarial parasites, and fungal and bacterial strains is discussed, along with recent updates in this area, with special emphasis on the structure–activity relationship.
FULL PAPERS
Thienopyrimidine-based agents bearing diphenylurea: Design, synthesis, and evaluation of antiproliferative and antiangiogenic activity
- First Published: 21 November 2022
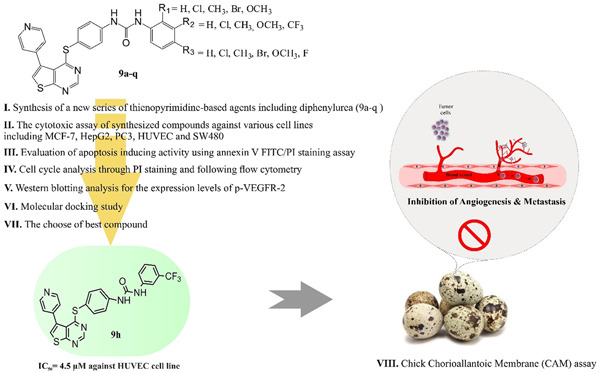
New thienopyrimidine derivatives were synthesized and evaluated as inhibitors of vascular endothelial growth factor receptor 2 (VEGFR-2). Most of the target compounds had antiproliferative activity against PC3, HepG2, MCF7, SW480, and HUVEC cells (9h: IC50 = 4.5–15.1 μM). In the chick chorioallantoic membrane assay, 9h effectively reduced the number of corresponding blood vessels.
From dithiocarbamates to branched dithiocarbazates: Compounds with potent antischistosomal activity
- First Published: 08 December 2022
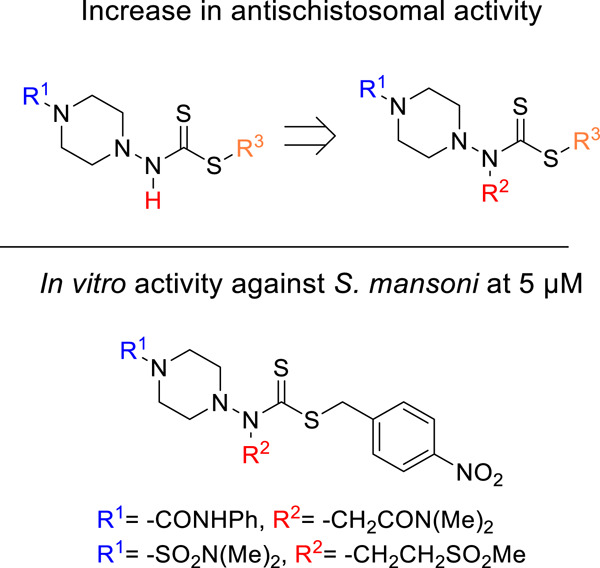
Four different series of dithiocarbazates and dithiocarbamates demonstrated the antischistosomal potential of this type of compound. After the N-aminopiperazine scaffold was identified as particularly promising, the in vitro antischistosomal activity could be increased to 5 µM by introducing a further substituent. The structurally related but synthetically more accessible 4-aminopiperidines showed higher cell toxicity or lower antischistosomal activity.
Synthesis and in vitro antiprotozoal evaluation of novel metronidazole–Schiff base hybrids
- First Published: 29 November 2022
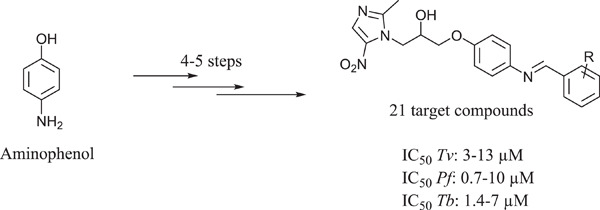
Twenty-one novel small molecules inspired by metronidazole and Schiff base compounds were evaluated against Trichomonas vaginalis and cross-screened against other pathogenic protozoans of clinical relevance. Compound 22 was identified as a broad-spectrum antiprotozoal agent, showing activities against all three pathogenic protozoans under investigation.
1H-1,2,3-Triazole−4H-chromene−D-glucose hybrid compounds: Synthesis and inhibitory activity against Mycobacterium tuberculosis protein tyrosine phosphatase B
- First Published: 23 November 2022
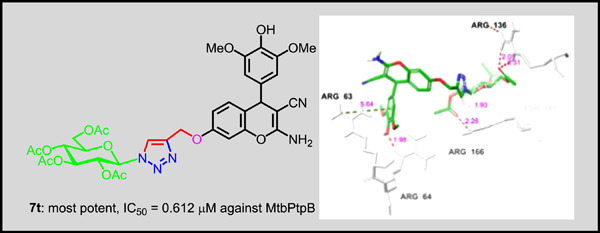
1H-1,2,3-Triazole−4H-chromene−D-glucose hybrid compounds 7a–w were synthesized and examined for their in vitro inhibition of Mycobacterium tuberculosis protein tyrosine phosphatase B. Compound 7t is the most potent inhibitor in vitro with IC50 = 1.23 μM. Induced-fit docking, MM-GBSA, and 300-ns MD simulation studies of 7t on the enzyme (PBD: 2OZ5) revealed the stability of the ligand-protein complex and insights into the mode of binding.
Synthesis, antimycobacterial, cytotoxicity, anti-inflammatory, in silico studies and molecular dynamics of pyrazole-embedded thiazolidin-4-one hybrids
- First Published: 02 December 2022
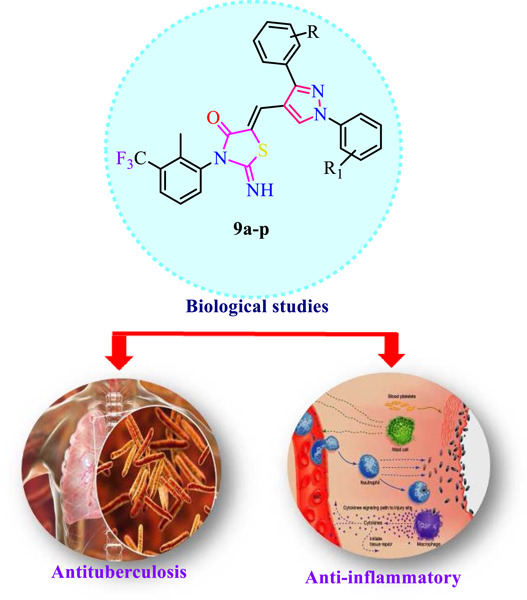
A new series of pyrazole embedded thiazolidin-4-one derivatives (9a–p) were developed to produce promising antitubercular leads. The in vitro antimycobacterial activity of the synthesized compounds was tested against replicating and nonreplicating Mtb H37Rv strains. Five compounds (9a, 9c, 9d, 9e, and 9f) emerged as promising antitubercular agents while being nontoxic to normal Vero cells.
Synthesis, computational and antimicrobial evaluation of some new pyridine derivatives
- First Published: 22 November 2022
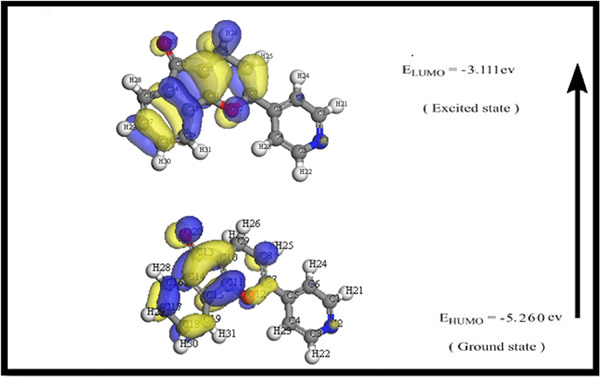
A series of pyridine compounds were prepared starting from 3-(dimethylamino)-1-(pyridin-4-yl)prop-2-en-1-one (14). Density function theory studies were made to establish the geometry of the tested compounds. Their antimicrobial activities were studied against Gram-positive and Gram-negative bacteria and two fungal species.
N-Substituted piperidine-3-carbohydrazide-hydrazones against Alzheimer's disease: Synthesis and evaluation of cholinesterase, beta-amyloid inhibitory activity, and antioxidant capacity
- First Published: 03 December 2022
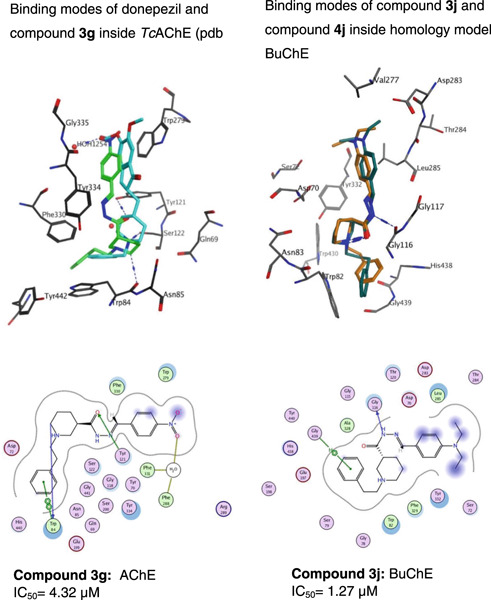
A series of piperidine-3-carbohydrazide-hydrazones bearing phenylethyl, phenylpropyl, and phenylbutyl substituents on piperidine nitrogen were designed and synthesized as acetyl-/butyrylcholinesterase (AChE/BuChE) inhibitors. Compounds 3g, 3j, and 4i have good AChE/BuChE and Aβ42 inhibitory potentials and antioxidant capacities and can therefore be suggested as promising multifunctional agents to combat Alzheimer's disease.
Design, synthesis, and structure–activity relationships of diindolylmethane derivatives as cannabinoid CB2 receptor agonists
- First Published: 27 November 2022
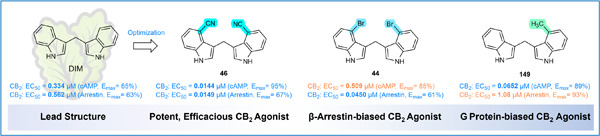
A series of diindolylmethane (DIM) derivatives were synthesized and evaluated for their affinities and functional potencies at the human cannabinoid (CB)1 and CB2 receptors. Compound 46 was found to be the most potent CB2 receptor agonist of the series. Compound 44 showed higher potency in β-arrestin than in cAMP accumulation assays, while 149 displayed a 19-fold bias for the G protein pathway. DIM and its analogs act as allosteric CB2 receptor agonists.
Design, synthesis, and biological evaluation of aminopyridine derivatives as novel tropomyosin receptor kinase inhibitors
- First Published: 18 November 2022
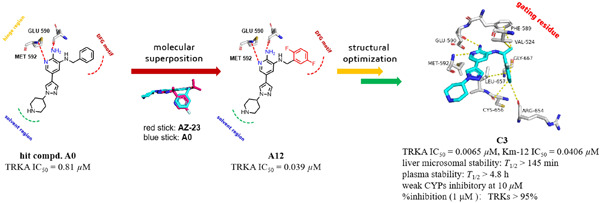
Tropomyosin receptor kinase (TRK) is a good target for the treatment of cancers caused by NTRK gene fusions. Based on rational drug design, 35 aminopyrimidine derivatives were designed, synthesized, and tested for TRKA inhibitory activity. Compounds C3, C4, and C6 are potent inhibitors of TRKA with IC50 values of 6.5, 5.0, and 7.0 nM, respectively. Compound C3 as a TRK inhibitor significantly inhibited the proliferation of KM-12 cells but not of MCF-7 and HUVEC cells.
Exploration of thiazolidine-2,4-diones as tyrosine kinase inhibitors: Design, synthesis, ADMET, docking, and antiproliferative evaluations
- First Published: 20 November 2022
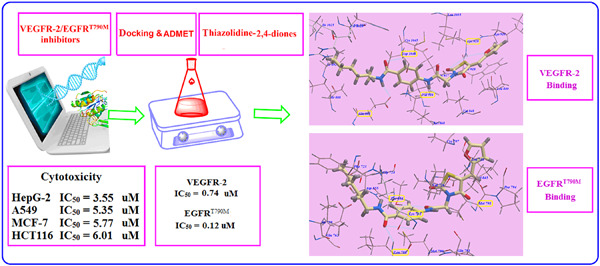
Twenty-two new thiazolidine-2,4-diones were modeled, constructed, and measured for their activity against four human cancer cell lines: HCT-116, MCF-7, A549, and HepG2. Molecular docking and MD simulation were performed to study the binding modes of the new congeners with the EGFR and VEGFR-2 receptors. Novel candidates were identified to construct better and binary VEGFR-2/EGFRT790M inhibitors with higher antitumor effects.
Design, synthesis, and biological evaluation of a potent PLK4 inhibitor WY29 with 1H-pyrazolo[3,4-d]pyrimidine scaffold
- First Published: 28 November 2022
![Design, synthesis, and biological evaluation of a potent PLK4 inhibitor WY29 with 1H-pyrazolo[3,4-d]pyrimidine scaffold](/cms/asset/2cdf1a9c-e3c4-41b8-a2b4-80d1bb38cb2f/ardp202200490-gra-0001-m.jpg)
Starting from the hit compound WY01, a series of Polo-like kinase 4 (PLK4) inhibitors with 1H-pyrazolo[3,4-d]pyrimidine core was designed and synthesized. Among them, compounds WY29, WY30, and WY31 exhibited outstanding in vitro enzyme inhibitory activity. At the cellular level, compound WY29 showed high antiproliferative activity against three breast cancer cell lines but weak inhibitory activity on a normal cell line.
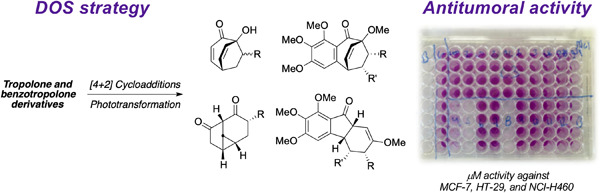
Synthesis and antitumoral evaluation of natural product-like compounds based on tropolone and benzotropolone derivatives
- First Published: 08 December 2022