Journal list menu
Export Citations
Download PDFs
Cover Picture
Macromol. Rapid Commun. 10/2012
- Page: 881
- First Published: 18 May 2012

Front Cover: Reversibly coagulatable and redispersible polyacrylate latexes are prepared by emulsion (co)poly-merization of methyl methacrylate (MMA) using a polymeric surfactant, PDMAEMA-block-PMMA. The resulting PMMA latexes can be readily coagulated with a trace amount of caustic soda. The coagulated latex pastes, after washing, can also be redispersed into fresh water to form stable emulsions by CO2 bubbling with ultrasonication. The coagulation and redispersion processes are repeatable. Further details can be found in the article by Q. Zhang, G. Yu, W.-J. Wang,* B.-G. Li, and S. Zhu* on page 916.
Back Cover
Macromol. Rapid Commun. 10/2012
- Page: 948
- First Published: 18 May 2012

Back Cover: Alkene and alkyne functionalized reactive polymers provide a facile strategy of surface modification that can readily proceed with promising thiol-ene and thiol-yne coupling reactions. Designer surfaces are demonstrated by low-protein fouling surfaces on silver, titanium, stainless steel, glass, PMMA, and PDMS substrates, as well as using cysteine-containing functional peptides to manipulate cell attachments. Further details can be found in the article by J.-T. Wu, C.-H. Huang, W.-C. Liang, Y.-L. Wu, J. Yu, and H.-Y. Chen* on page 922.
Masthead
Contents
Review
Alkene Metathesis – A Tool for the Synthesis of Conjugated Polymers
- Pages: 886-910
- First Published: 17 April 2012
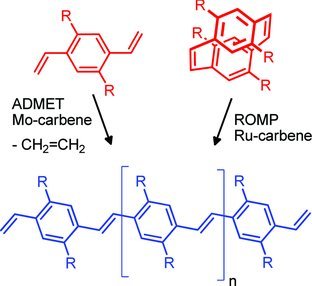
Alkene metathesis is powerful in the synthesis of vinyl-bridged conjugated polymers. Using either ring-opening metathesis polymerization (ROMP) or ADMET (acyclic diene metathesis) poly(paraphenylenevinylene)s (PPVs), and polyacetylenes (PAs) can be prepared efficiently. The influence of monomer, catalyst, and choice of method upon the properties of the formed polymers are discussed in this review.
Communications
Surface-Modifiable Free-Floating Films Formed by Multiway Connection of Collagen-Like Triple-Helical Peptides
- Pages: 911-915
- First Published: 05 March 2012
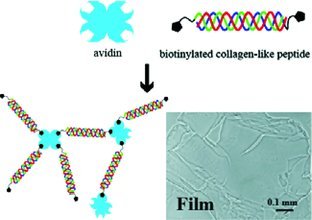
Square-millimeter-sized free-floating translucent films are formed in physiological buffer by multiway connections between biotinylated collagen-like triple-helical peptides and avidin. The film thicknesses can be controlled by the concentrations of the components, and the surfaces can be further modified by taking advantage of exposed biotin (or avidin) functionalities.
Preparation of CO2/N2-Triggered Reversibly Coagulatable and Redispersible Polyacrylate Latexes by Emulsion Polymerization Using a Polymeric Surfactant
- Pages: 916-921
- First Published: 05 April 2012
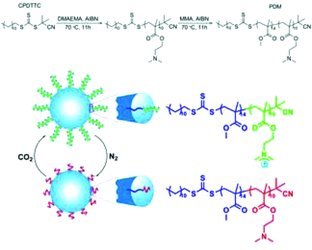
A polymeric surfactant (PDMAEMA-block-PMMA) was used to prepare reversibly coagulatable and redispersible PMMA latexes through RAFT (co)polymerization. The emulsions could be coagulated by N2 bubbling with gentle heating. The coagulated latex pastes after washing could also be redispersed into fresh water to form stable emulsions by CO2 bubbling with ultrasonication. The coagulation and redispersion processes were repeatable.
Reactive Polymer Coatings: A General Route to Thiol-ene and Thiol-yne Click Reactions
- Pages: 922-927
- First Published: 20 February 2012
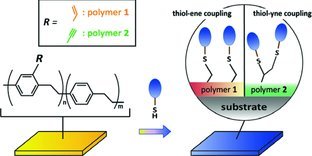
Vapor-deposited reactive polymer coatings provide a general route to support thiol-ene and thiol-yne reactions on a variety of substrate materials. Surface functions can be activated through a simple design of thiol-terminated molecules, and the corresponding responses are efficiently and accurately manipulated.
Inverse Suspension Polymerization as a New Tool for the Synthesis of Ion-Imprinted Polymers
- Pages: 928-932
- First Published: 21 February 2012
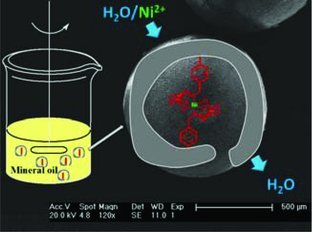
Inverse suspension polymerization is used for the first time to produce ion- imprinted polymer beads. This facile approach leads to materials with high site accessibility and large adsorption capacities. The ratio between the functional monomer and the crosslinker plays an important role on the “imprinting effect” of the polymers.
Facile Preparation of Polymeric Dimers from Amphiphilic Patchy Particles
- Pages: 933-937
- First Published: 27 February 2012
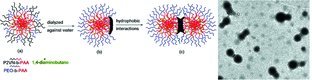
Uniform polymeric dimers are efficiently obtained via hydrophobic interactions from amphiphilic mixed-shell micelles under optimized conditions in selective solvent. These micelles are fabricated by non-covalent crosslinking methods. The results are fully characterized by scanning electron microscopy, transmission electron microscopy, dynamic light scattering, and Fourier transform infrared spectroscopy.
In Situ Hetero End-Functionalized Polythiophene and Subsequent “Click” Chemistry With DNA
- Pages: 938-942
- First Published: 21 February 2012
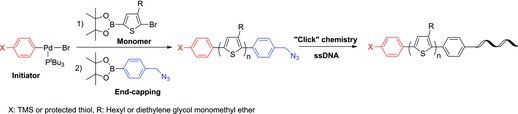
A versatile synthetic approach for bifunctionalized polythiophenes is described. The active end-group further reacts with DNA via “click” chemistry to form a polythiophene/DNA hybrid structure. Engineering the end functionalization is meant not only to allow for the synthesis of novel block copolymers, but also to be a platform for molecular level study.
Estimation of Self-Diffusion Coefficients of Small Penetrants in Semicrystalline Polymers Using Single-Sided NMR
- Pages: 943-947
- First Published: 02 March 2012
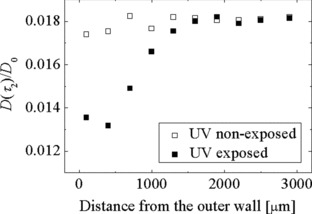
An efficient way to measure proton self-diffusion coefficients (D) of small penetrant molecules in semicrystalline polymers with different shapes and sizes and by using unilateral NMR is introduced. One-dimensional profiles of D across plates of semicrystalline polymers with several millimeter thickness and a heterogeneous structure are reported for the first time.






