Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
REVIEW
Depressive Disorder Affects TME and Hormonal Changes Promoting Tumour Deterioration Development
- Pages: 403-418
- First Published: 08 May 2025
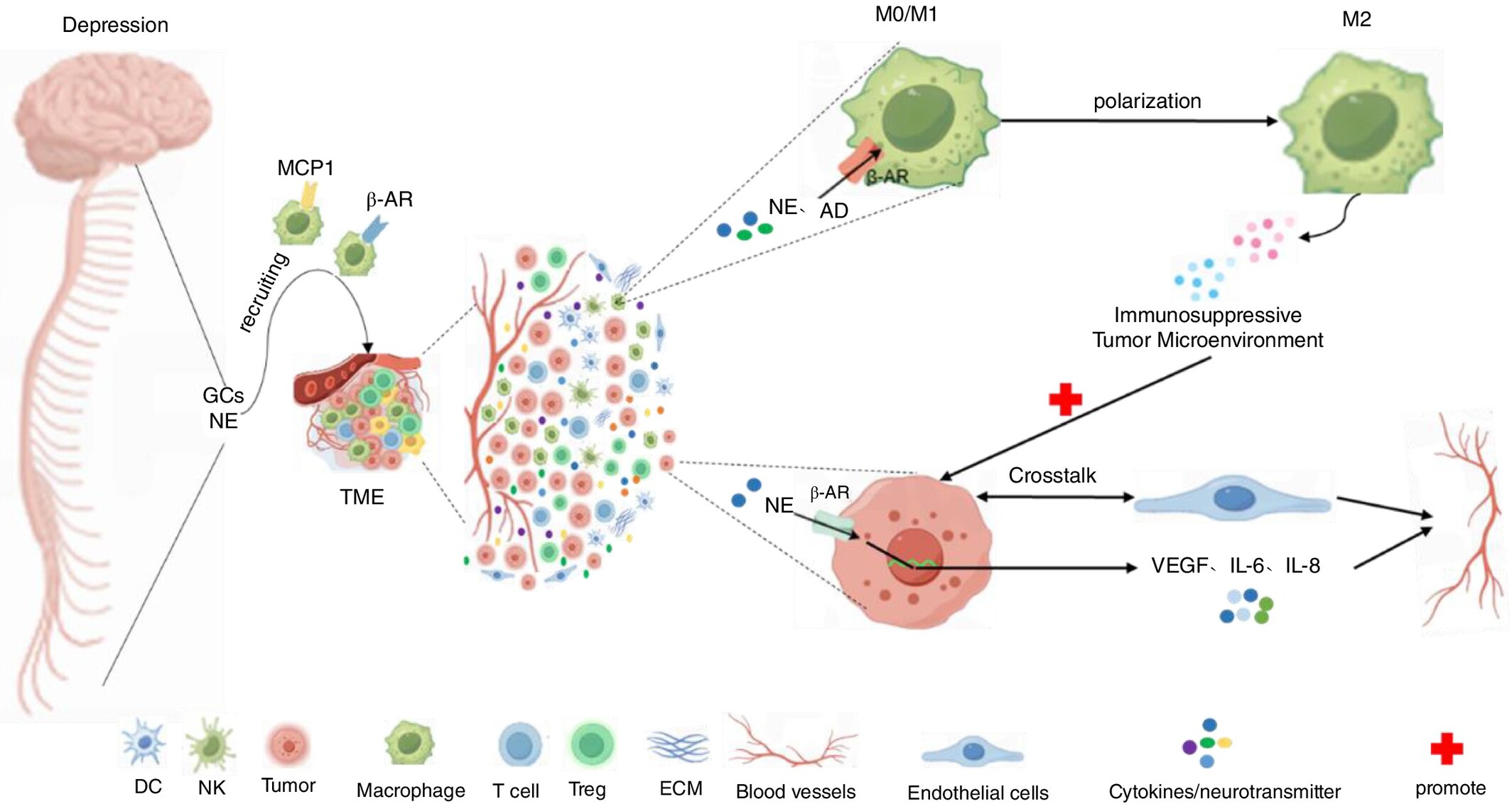
Cancer patients are often accompanied by depression, and the presence of depression can inversely promote the deterioration of cancer patients' conditions and thus affect the survival rate of cancer patients. This may be related to the triggering of the HPA axis, the imbalance of the SNS system, intestinal flora disorders, and other pathways leading to the release of some glucocorticoids, inflammatory factors, neurotransmitters, and other dysregulation caused by the subtle changes in the relationship between the immune system and the tumour microenvironment (TME), which leads to the immune-suppressive microenvironment. For example, the function and killing effect of the relevant immune cells CD4, CD8+T cells, NK cells, etc. are weakened, and the immunosuppressive class of cells, Tregs cells, is increased or T cells are depleted leading to tumour immune escape. Depression can induce peripheral macrophage infiltration in the TME and polarisation toward tumour-associated macrophages (M2 phenotype), which ultimately promotes tumour immunosuppression leading to cancer progression.
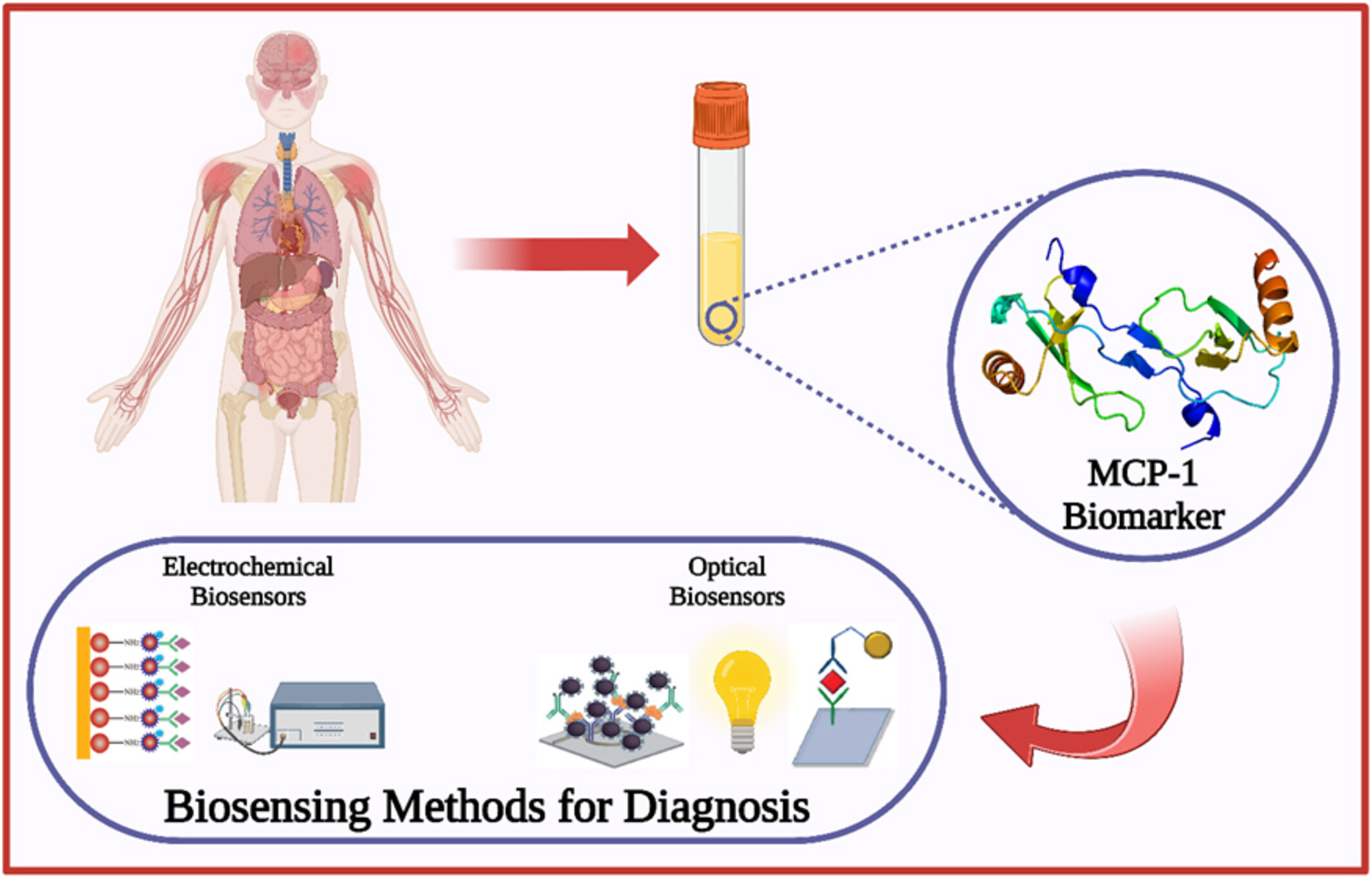
A Review on Biosensors for Quantification of MCP-1 as a Potential Biomarker in Diseases
- Pages: 419-433
- First Published: 14 May 2025
ORIGINAL ARTICLE
Non-Antigen-Specific B Cells Induced Regulatory CD4+ T Cells Through Decreasing T Cell Activation
- Pages: 434-443
- First Published: 04 May 2025
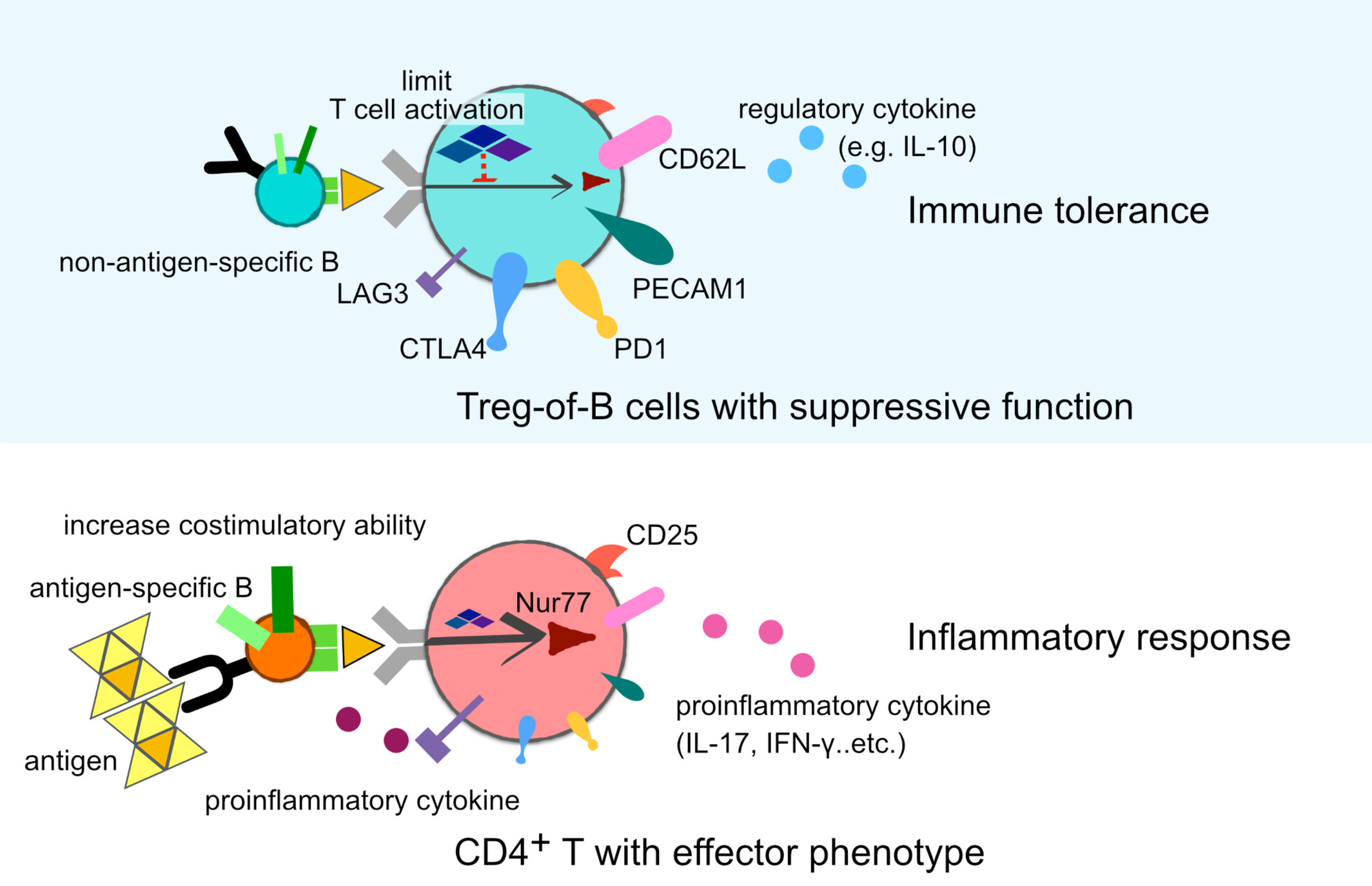
Naïve B cells elicit Treg-of-B cells with suppressive functions by limiting T cell activation during differentiation. Treg-of-B cells express various immunoregulation-associated molecules and regulatory cytokines. Conversely, activated antigen-specific B cells induce effector T cells through costimulatory molecules and elevated proinflammatory cytokines.
Increased CD8dim and Decreased CD8bright T Cells as Immunological Signature for Multibacilary Leprosy Patients
- Pages: 444-452
- First Published: 05 May 2025
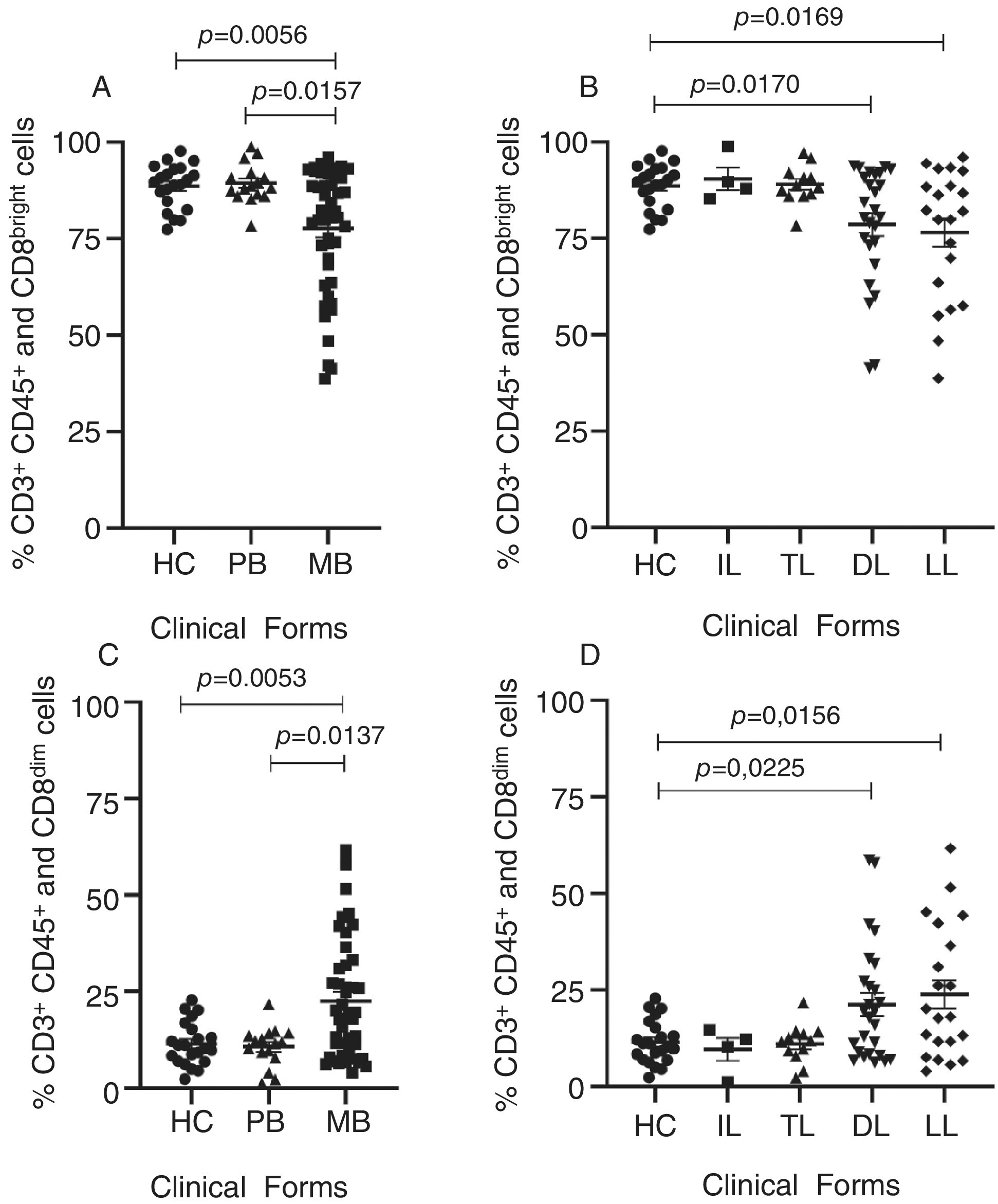
Leprosy, a chronic infectious disease caused by Mycobacterium leprae, manifests in a spectrum of clinical forms and severity. This study investigated the percentage of CD8+ T cells and their subpopulations (CD8bright and CD8dim T cells) in leprosy patients stratified by clinical forms, bacterial load, and age. An increased percentage of CD8dim T cells and a decreased percentage of CD8bright T cells were associated with severe multibacillary and lepromatous forms of leprosy, independent of bacillary load and age of patients. These findings suggest CD8bright and CD8dim T cell profiles are critical indicators of severity in leprosy, highlighting their potential as biomarkers for clinical evaluation.
Antibiotic-Induced Dysbiosis of the Gut Microbiota Shifts Host Tryptophan Metabolism and Increases the Susceptibility of Mice to Pulmonary Infection With Pseudomonas aeruginosa
- Pages: 453-466
- First Published: 19 May 2025
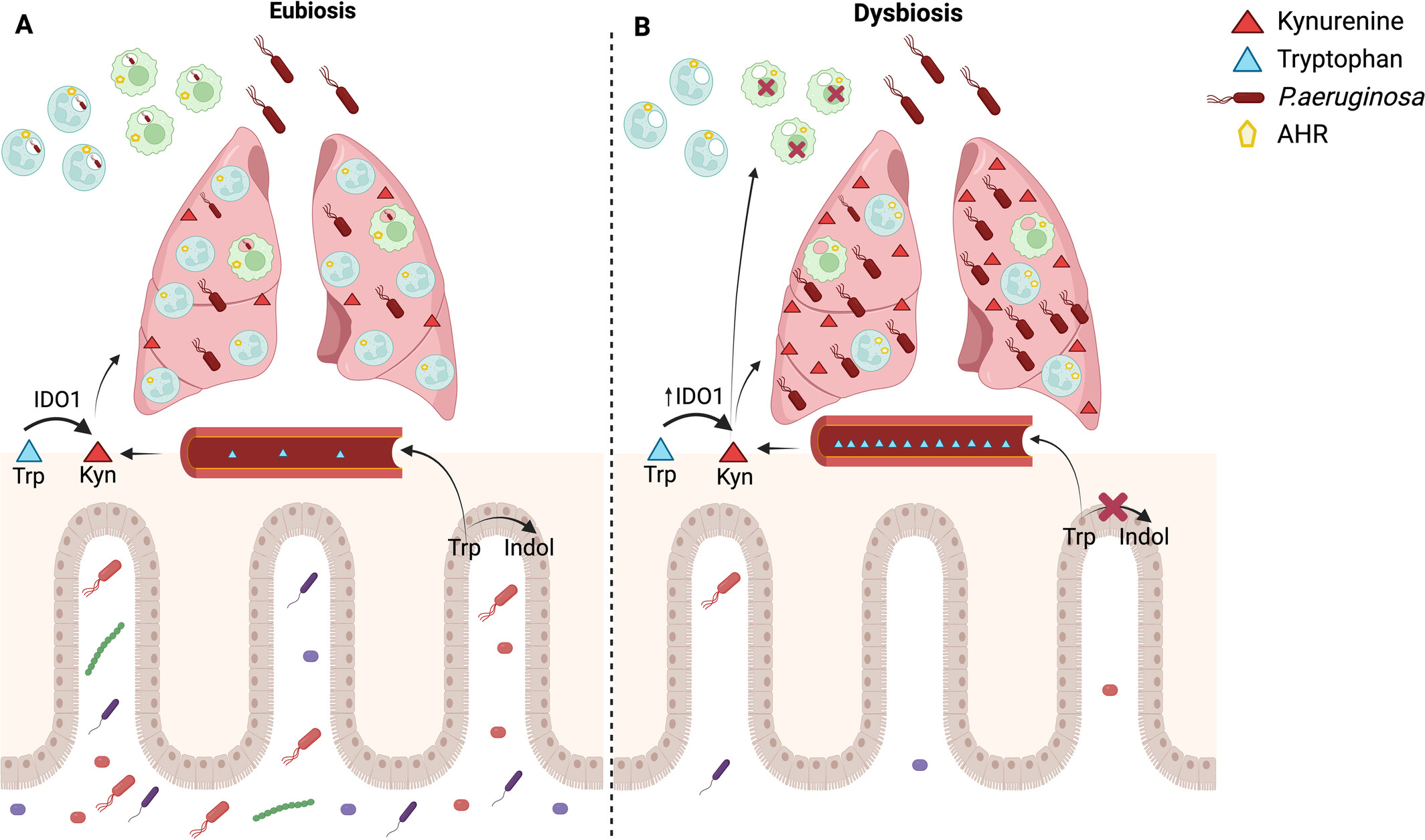
Antibiotic-induced dysbiosis of the gut microbiota shifts host tryptophan metabolism and impairs the innate immune response during Pseudomonas aeruginosa pulmonary infection. (A) Eubiosis: During eubiosis, the intestinal microbiota metabolises tryptophan into indole metabolites, which keeps tryptophan levels within normal serum concentrations. However, during a Pseudomonas aeruginosa infection, an inflammatory response induces tryptophan to be converted into kynurenine. This leads to an increase in kynurenine concentration in the lungs. Once P. aeruginosa is recognised by resident cells, alveolar macrophages will phagocytose the bacteria, initiating an inflammatory response that results in the massive recruitment of neutrophils. Neutrophils will then phagocytose and eliminate the bacteria, effectively controlling the bacterial load in the lungs. (B) Dysbiosis: The antimicrobial cocktail induces dysbiosis in the intestinal microbiota, decreasing bacterial groups that metabolise tryptophan into indole. This process causes an elevation in serum tryptophan levels. In response to an infectious stimulus, there is an increase in the activity of the enzyme IDO1, which raises kynurenine concentrations in the lungs. The kynurenine activates the AHR receptor, which hinders the phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. This impairment in phagocytosis results in a decreased influx of neutrophils. Additionally, the neutrophils that are recruited exhibit increased expression of AHR, which can also be activated by excess kynurenine, further disrupting the ability to phagocytose the bacteria. Created in BioRender. Brito (2025) https://BioRender.com/z10g943.
CCR5-Mediated Reprogramming of Regulatory T Cells and Monocytic-Myeloid-Derived Suppressor Cells in Young Dyslipidemic Individuals: A Plausible Therapeutic Approach
- Pages: 467-481
- First Published: 19 May 2025
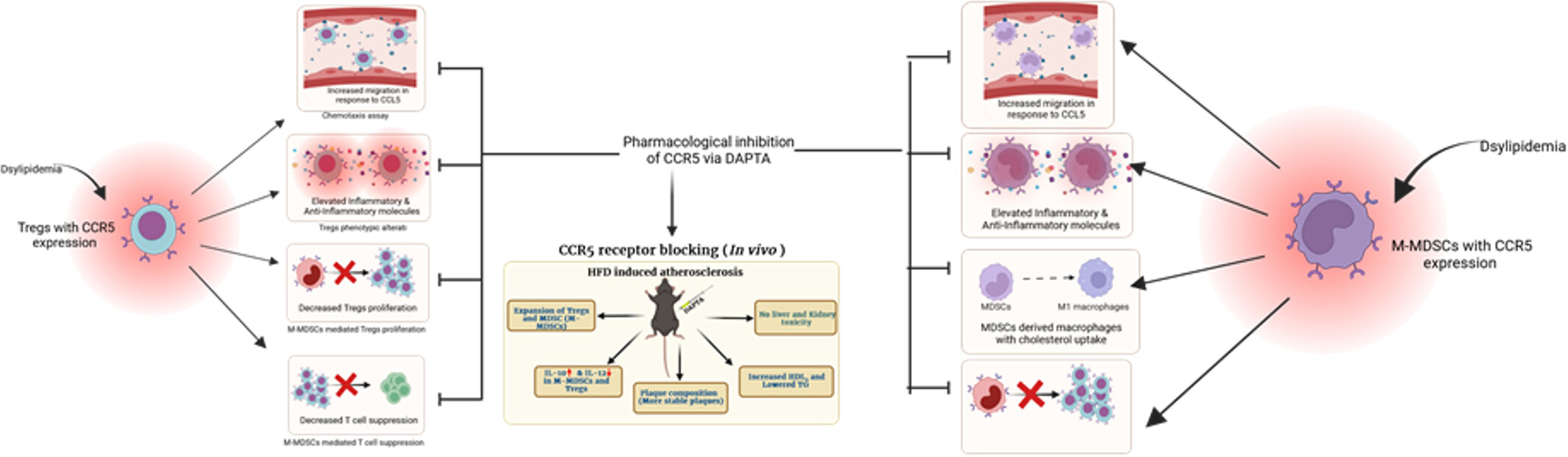
Inflammatory milieu upregulates CCR5 expression on Tregs and M-MDSCs of young and naive dyslipidemic individuals (DLP), altering their immunosuppressive phenotype and functionality. Inhibition of CCR5 by DAPTA restores their immunosuppressive phenotype and functionality as well as reduces the plaque inflammation in a mouse model of atherosclerosis.
Beyond Canonical Immune Checkpoints: Overexpression of TNFRSF Members 4-1BB and OX-40 Marks T Cells Exhibiting Phenotypic Features of Exhaustion in Cervical Carcinoma
- Pages: 482-500
- First Published: 19 May 2025
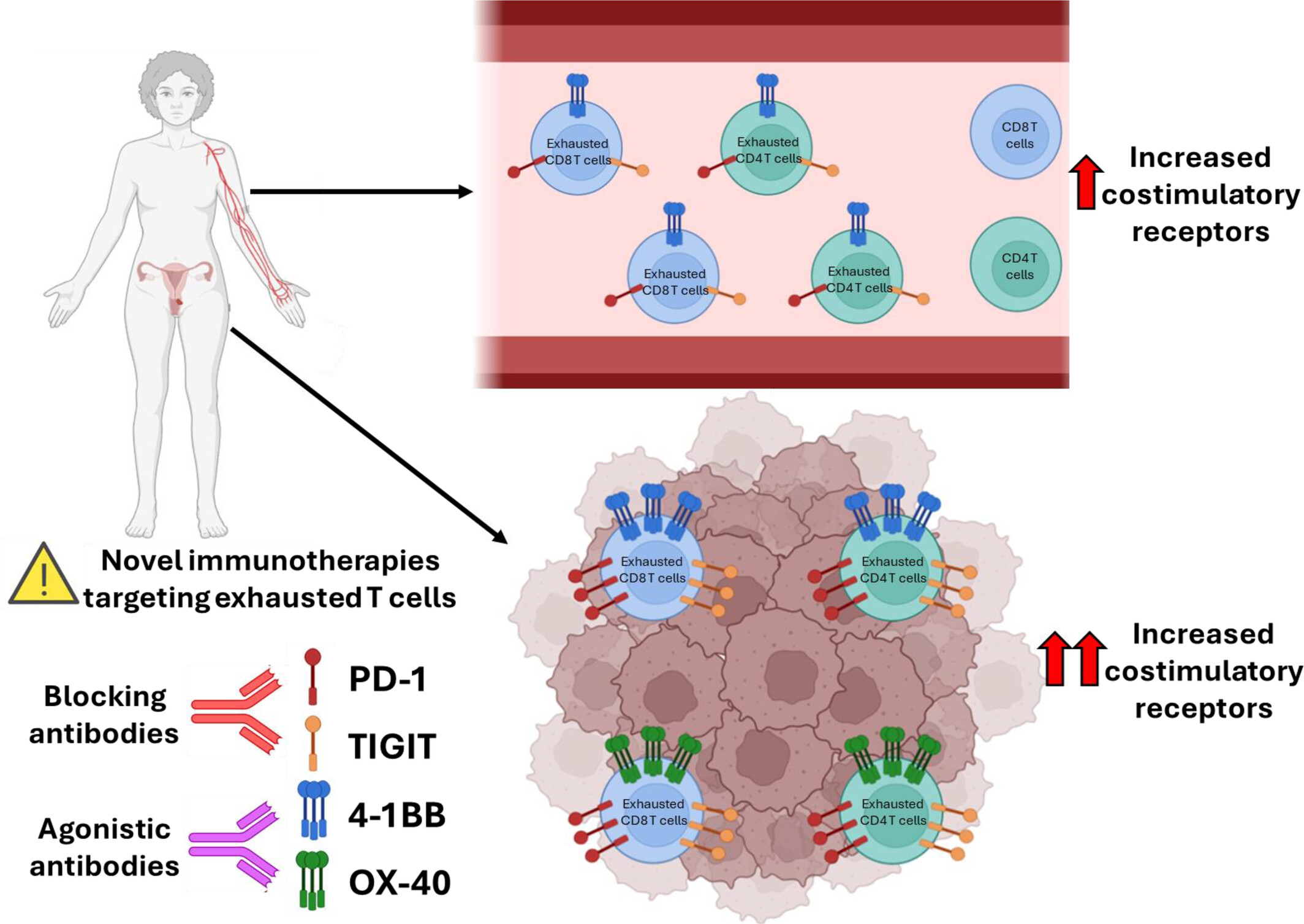
Phenotypically exhausted T cells are enriched in patients with cervical cancer. Tumour-infiltrating T cells exhibit marked overexpression of PD-1, a key marker of T cell exhaustion. Notably, co-stimulatory receptors 4-1BB and OX-40 are selectively upregulated in these exhausted T cells, highlighting them as promising targets to restore anti-tumour immunity.
BCG Vaccination Reprograms the Function of M-MDSCs and Aggravates Necrotizing Enterocolitis in Neonates
- Pages: 501-517
- First Published: 21 May 2025
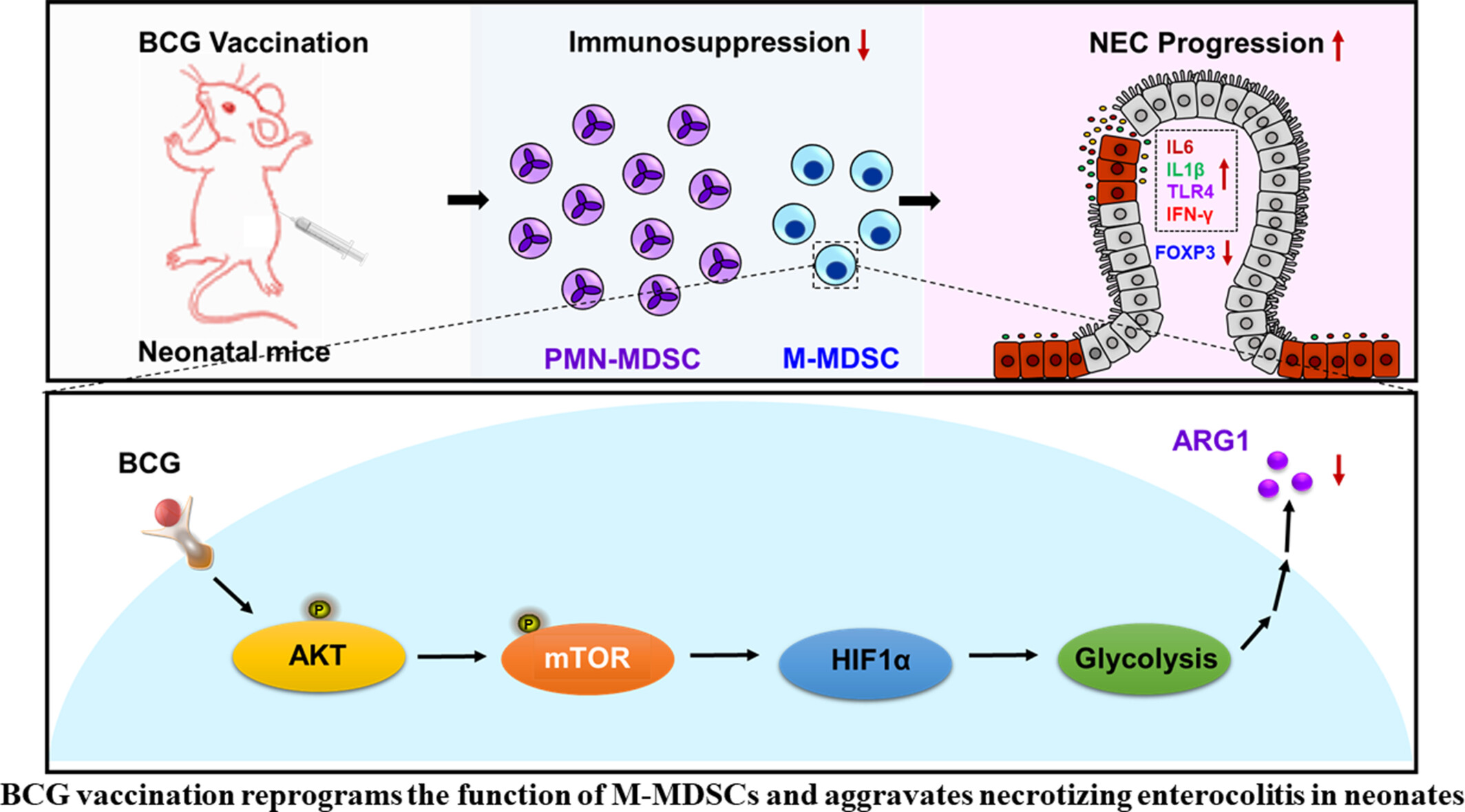
This study demonstrates that BCG vaccination exacerbates necrotizing enterocolitis (NEC) by enhancing glycolysis and mTOR-HIF1α signalling in monocytic MDSCs, impairing their immunosuppressive function. Inhibiting these pathways restored MDSC activity and reduced NEC severity, revealing a mechanism for BCG's off-target effects. The findings highlight the need for cautious BCG use in preterm infants to avoid disrupting immune tolerance and increasing NEC risk.
Limitation of Deleterious iNOS-Expressing Interstitial Macrophages Is Dependent on Protective IL-1α and IL-17 in Tuberculosis and Existing Obesity
- Pages: 518-533
- First Published: 21 May 2025
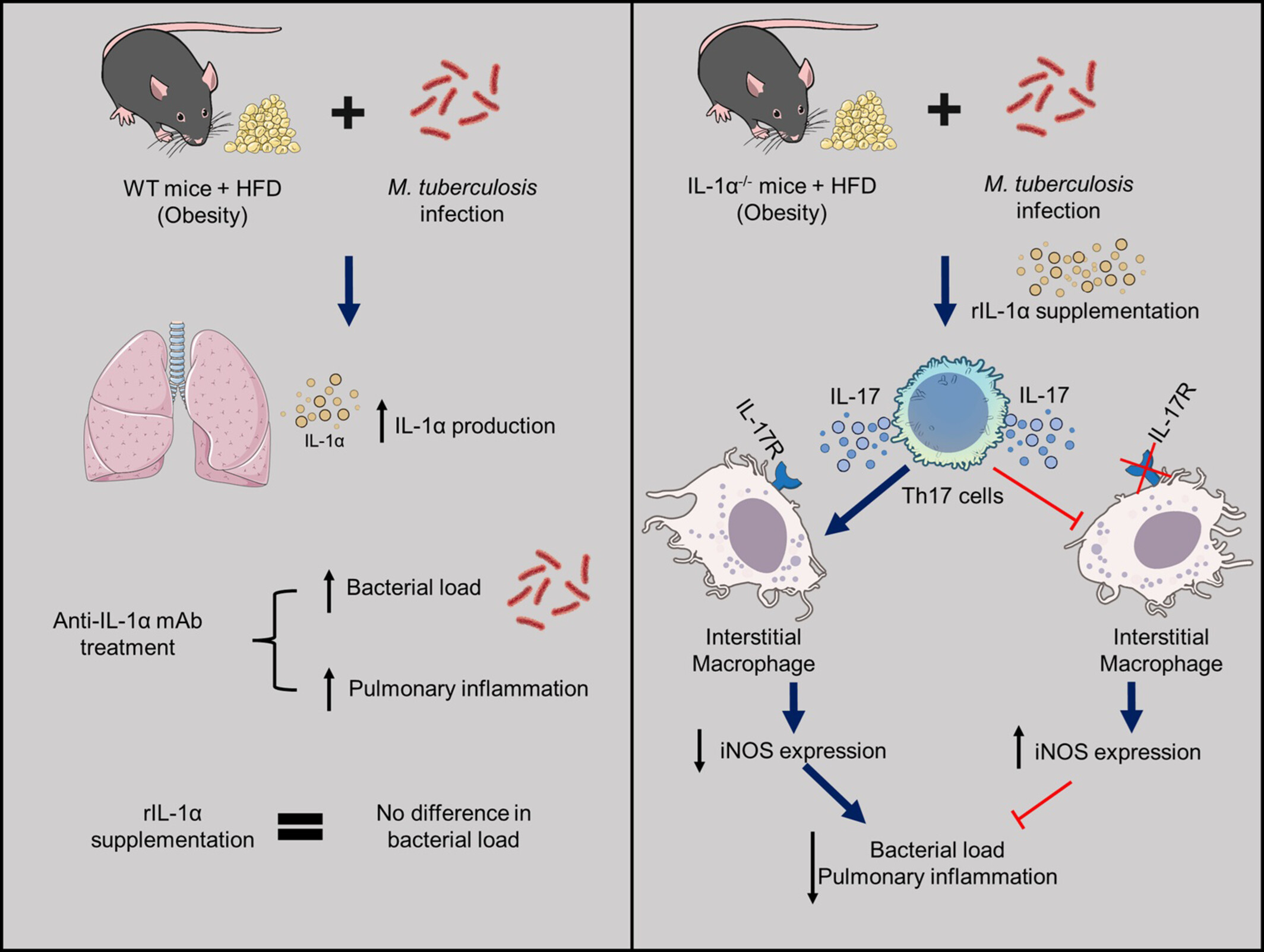
The pathogenesis of tuberculosis with existing obesity that aggravates pulmonary inflammation remains unclear. IL-1α is protective because the deficiency of this cytokine or anti-IL-1α monoclonal antibody treatment aggravates the infection and lung inflammation. IL-1α supplementation in IL-1α deficient mice (IL-1α−/−) increases Th17 cells (CD4+IL-17+ cells), which negatively regulate iNOS-expressing interstitial macrophages, with consequent reduction of bacterial load and inflammation in the lungs. The subsequent impairment of the IL-17–IL-17R axis abolishes the protection conferred by rIL-1α, causing an increase in iNOS-expressing interstitial macrophages and bacterial load.
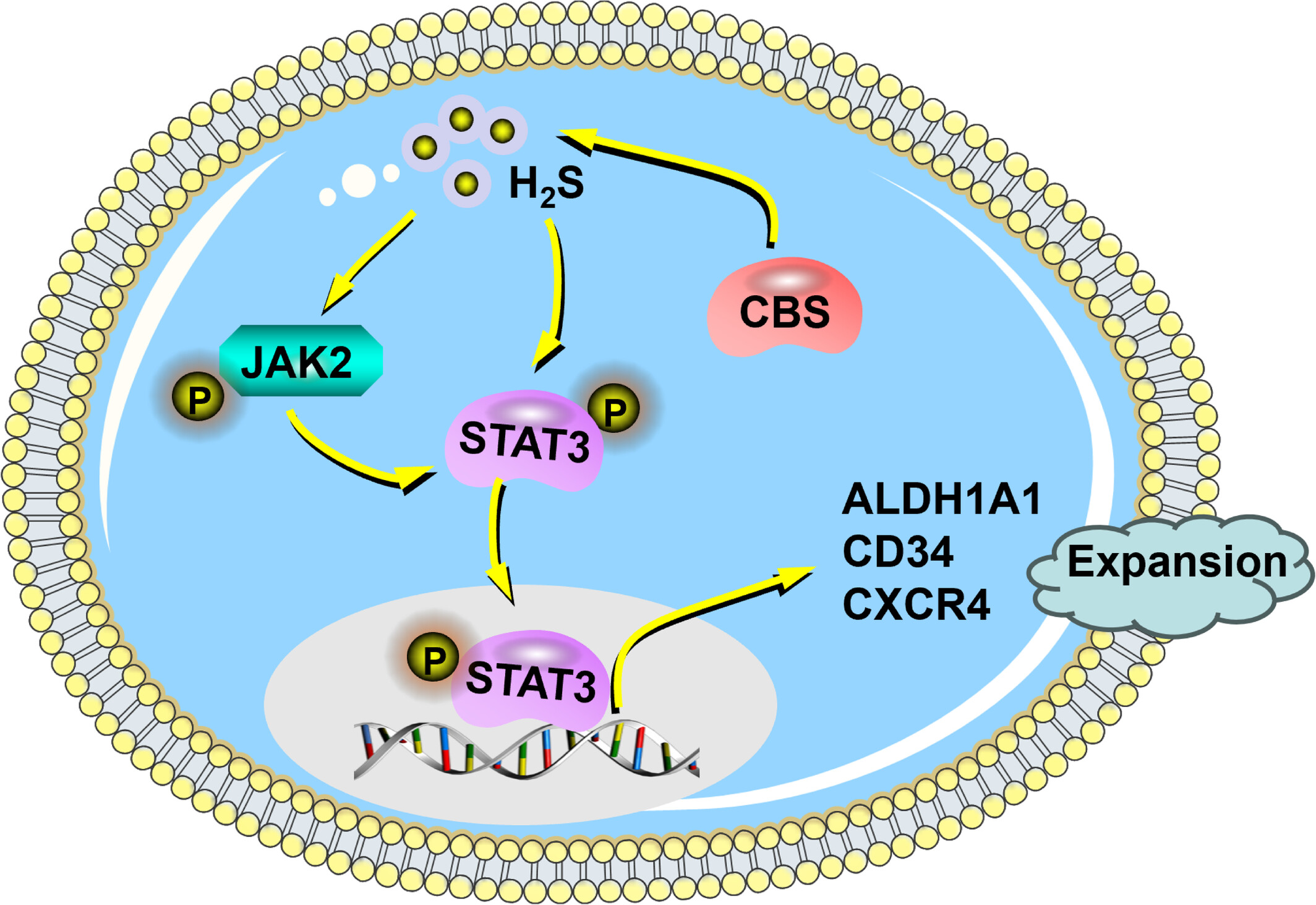
Endogenous Hydrogen Sulphide Promotes the Ex Vivo Expansion of Haematopoietic Stem Cells by Regulating the Activation of the JAK2/STAT3 Pathway
- Pages: 534-543
- First Published: 22 May 2025