Journal list menu
Export Citations
Download PDFs
Issue Information
Full Papers
Polyaniline-anchored palladium catalyst-mediated Mizoroki–Heck and Suzuki–Miyaura reactions and one-pot Wittig–Heck and Wittig–Suzuki reactions
- Pages: 1-6
- First Published: 06 November 2014
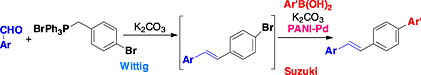
A polyaniline-anchored palladium heterogeneous catalyst was prepared and screened for coupling reactions of aryl halides. The robust, recyclable catalyst was effective in Mizoroki–Heck and Suzuki–Miyaura reactions of aryl bromides and iodides. The catalyst system was further employed for one-pot Wittig–Heck and Wittig–Suzuki combinations to obtain conjugated compounds in good conversions.
Comparison between various Keggin and Wells–Dawson sandwich-type polyoxometalates in catalytic oxidation of cyclooctene and cyclohexene with hydrogen peroxide
- Pages: 7-11
- First Published: 23 October 2014

Tetra-n-butylammonium salts of [M4(PW9O34)2]m− and [M4(P2W15O56)2]n− were used as effective catalysts for the oxidation of cyclooctene and cyclohexene under various conditions. Wells–Dawson-type polyoxometalates were less active than Keggin ones. Higher conversions were obtained with Zn- and slightly lower with Fe-substituted polyoxometalates of both sandwich-type compounds.
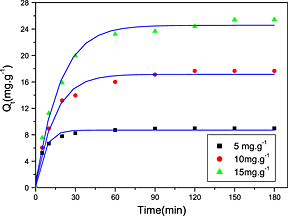
Metal–organic framework MIL-125(Ti) for efficient adsorptive removal of Rhodamine B from aqueous solution
- Pages: 12-19
- First Published: 23 October 2014
Binuclear dichlorido(η6-p-cymene)ruthenium(II) complexes with bis(nicotinate)- and bis(isonicotinate)-polyethylene glycol ester ligands
- Pages: 20-25
- First Published: 06 November 2014

Neutral binuclear and mononuclear ruthenium complexes with bis(nicotinate)- or bis(isonicotinate)-polyethylene glycol esters and ethylene glycol monomethyl ether nicotinate, respectively, were synthesized and characterized. Stability of the binuclear complexes in the presence of DMSO was studied, as well as the formation of a cationic complex. Ligand precursors and ruthenium(II) complexes were tested for their in vitro cytotoxicity against five tumour cell lines.
Green and effective route for the synthesis of monodispersed palladium nanoparticles using herbal tea extract (Stachys lavandulifolia) as reductant, stabilizer and capping agent, and their application as homogeneous and reusable catalyst in Suzuki coupling reactions in water
- Pages: 26-32
- First Published: 17 November 2014
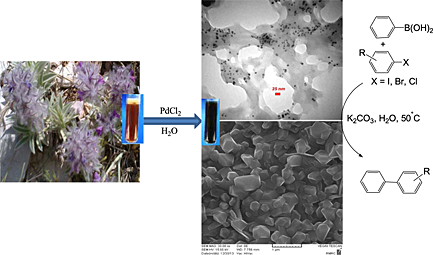
Palladium nanoparticles supported on cucurbit[6]uril: an efficient heterogeneous catalyst for the Suzuki reaction under mild conditions
- Pages: 33-39
- First Published: 02 December 2014
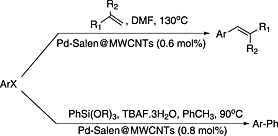
Pd(II) salen complex covalently anchored to multi-walled carbon nanotubes as a heterogeneous and reusable precatalyst for Mizoroki–Heck and Hiyama cross-coupling reactions
- Pages: 40-44
- First Published: 19 November 2014
Iron phthalocyanine as new efficient catalyst for catalytic transfer hydrogenation of simple aldehydes and ketones
- Pages: 45-49
- First Published: 17 November 2014
CTH reaction of differently substituted aldehydes and ketones were studied using iron phthalocyanine catalyst, in order to alternate the typically used rare transition metals (Ir, Rh, Ru) by easily available and less expensive metal. During this study iron phthalocyanine was proven to be an efficient hydrogenation catalyst and the anchored version of it was also prepared. The immobilized iron phthalocyanine showed similar activity in CTH reaction of carbonyl compounds and it was easy to handle and possible to recycle.
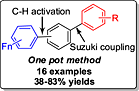
One-pot synthesis of polyfluoroterphenyls via palladium-catalyzed Suzuki–Miyaura coupling of chlorobromobenzene and CH bond functionalization of perfluoroarenes
- Pages: 50-56
- First Published: 17 November 2014
Book Reviews
Keynotes in Organic Chemistry, 2nd edition, Andrew F. Parsons, John Wiley & Sons; December 2013, 300 pages, ISBN:
978-1-119-99914-0 , Hardcover £80.00, E-book£19.99
- Page: 57
- First Published: 17 November 2014
THOMAS A. ALBRIGHT, JEREMY K. BURDETT and MYUNG-HWAN WHANGBO (Editors) Orbital Interactions in Chemistry, Wiley-Blackwell, 2nd edition, 2013, 834 pp. Price £113.00. ISBN-10: 047108039X. ISBN-13: 978-0471080398
- Page: 58
- First Published: 06 November 2014
Nanomaterials in Catalysis, Philippe Serp and Karine Philippot (editors), Wiley-VCH, 2013, 516 pages, ISBN: 978-3-527-33124-6
- Page: 59
- First Published: 19 November 2014
The Organometallic Chemistry of the Transition Metals, 6th edition Robert H. Crabtree Wiley, 2014 520 pages €80.40 £66.95 ISBN: 978-1-118-13807-6
- Page: 60
- First Published: 14 November 2014





![Palladium nanoparticles supported on cucurbit[6]uril: an efficient heterogeneous catalyst for the Suzuki reaction under mild conditions](/cms/asset/cd33483f-90d6-4d9c-ab94-a57ce93e4869/aoc3245-toc-0001.png)