Journal list menu
Export Citations
Download PDFs
Full Papers
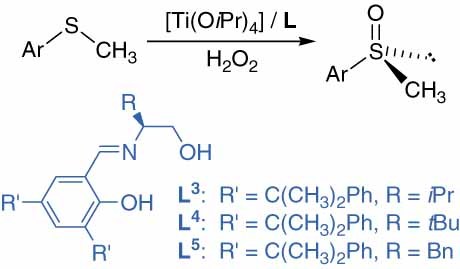
Asymmetric oxidation of sulfides with H2O2 catalyzed by titanium complexes of Schiff bases bearing a dicumenyl salicylidenyl unit
- Pages: 325-330
- First Published: 09 February 2011
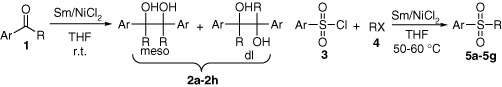
Novel synthesis of pinacols and sulfones promoted by SmNiCl2 bimetallic system
- Pages: 331-334
- First Published: 17 February 2011
Bis[(L)prolinato-N,O]Zn in acetic acid–water: a novel catalytic system for the synthesis of β-amino carbonyls via Mannich reaction at room temperature
- Pages: 335-340
- First Published: 26 January 2011
![Bis[(L)prolinato-N,O]Zn in acetic acid–water: a novel catalytic system for the synthesis of β-amino carbonyls via Mannich reaction at room temperature](/cms/asset/4ae373e1-2895-4726-b79a-179e8a4347b9/mgra001.jpg)
The Mannich reaction is a valuable coupling protocol for preparing amino carbonyl compounds. Chemistry has made significant advances in this field, but some challenges remain. Recently developed methods for carrying out this reaction in water as a reaction media are reviewed, and viewed from the perspective of green chemistry.
N2,N2′-disubstituted oxalic acid bishydrazides: novel ligands for copper-catalyzed CN coupling reactions in water
- Pages: 341-347
- First Published: 09 February 2011
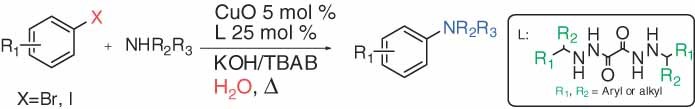
A series of N2,N2′-disubstituted oxalic acid bishydrazides were been synthesized and applied to be novel and effective ligands for copper-catalyzed Ullmann- type CN coupling reaction in water. A variety of amines could be effectively N-arylated with aryl halides under both microwave irradiation and conventional heating (even at 30 °C) with good to excellent yields.
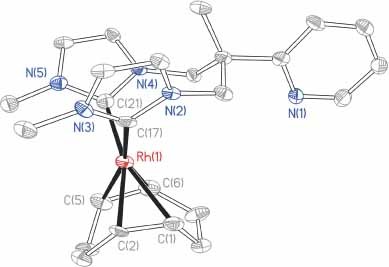
Synthesis, characterization, antimicrobial, SOD mimic and DNA interaction behavior of copper(II) complexes with pefloxacin and phenanthroline derivatives
- Pages: 348-355
- First Published: 09 February 2011
Anion exchange in trimethyl- and triphenyltin complexes with chromogenic ligands: solution equilibria and colorimetric anion sensing
- Pages: 356-365
- First Published: 25 March 2011
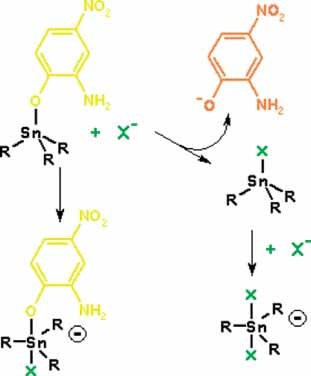
Substitution of a chromogenic ligand in triorganotin compounds by anions leads to strong color change and can be used for determination of equilibrium constants for anion exchange and addition reactions and for colorimetric anion sensing in polar organic solvents with selectivity AcO− > F− > H2PO4− > Cl− ≫ Br−.
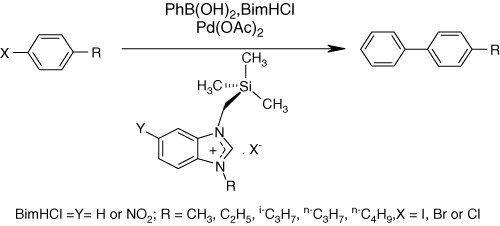
Synthesis, microwave-promoted catalytic activity in Suzuki–Miyaura cross-coupling reactions and antimicrobial properties of novel benzimidazole salts bearing trimethylsilyl group
- Pages: 366-373
- First Published: 04 April 2011
Novel quasi-scorpionate ligand structures based on a bis-N-heterocyclic carbene chelate core: synthesis, complexation and catalysis
- Pages: 374-382
- First Published: 29 March 2011
Syntheses and properties of ethoxylated double-tail trisiloxane surfactants containing a propanetrioxy spacer
- Pages: 383-389
- First Published: 29 March 2011
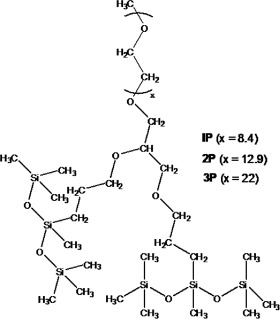
The synthesis, characterization, surface activity and hydrolysis-resistant ability of a new type of ethoxylated double-tail trisiloxane surfactants containing a propanetrioxy spacer are described. The trisiloxane surfactant 1P possesses a good spreading ability on parafilm and Ficus microcarpa leaf surfaces, and its aqueous solutions are stable for 130 days in an acidic environment (pH 4.0) with surface tension values less than 23 mN m−1.
Synthesis of new boron complexes: application to transfer hydrogenation of acetophenone derivatives
- Pages: 390-394
- First Published: 04 April 2011
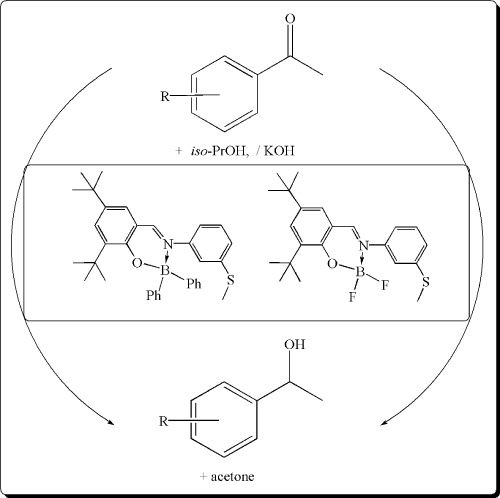
Two new boron complexes were synthesized from N-[3-(methylmercapto)aniline]-3,5-di-tert-butylsalicylaldimine (LH) with boron reagent BPh3 or BF3.Et2O. All new boron complexes were fully characterized by both analytical and spectroscopic methods. The catalytic activites of complexes [LBPh2], 2, and [LBF2], 3, in the transfer hydrogenation of acetophenone derivatives were tested. The stable boron complexes were found to be efficient catalysts in the transfer hydrogenation of aromatic ketones in good conversions up to 99% in the presence of iso-PrOH/NaOH.
A reusable CuSO4 · 5H2O/cationic 2,2′-bipyridyl system catalyzed homocoupling of terminal alkynes in water
- Pages: 395-399
- First Published: 25 March 2011
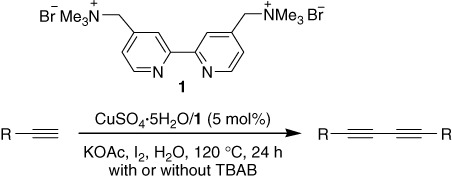
A reusable CuSO4·5H2O/cationic 2,2′-bipyridyl system catalyzed the homocoupling reaction of terminal alkynes in water using I2 as the additive in the presence or absence of TBAB, giving the 1,3-diynes in good to high yields. After reaction, the residual aqueous solution could be reused several times.
Communication
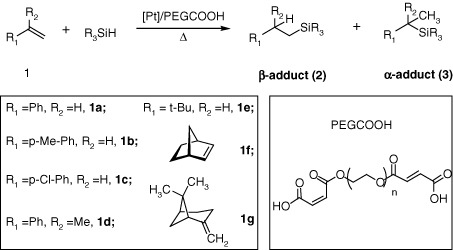
Use of carboxylated polyethylene glycol as promoter for platinum-catalyzed hydrosilylation of alkenes
- Pages: 400-405
- First Published: 04 April 2011