Photocatalyzed Annulation-Biselenylation of Enynone with Diarylselenides toward Biselenium-Substituted 1-Indanones under Metal- and Photosensitizer-Free Conditions
Corresponding Author
Hang-Dong Zuo
School of Safety Science and Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorHua-Feng Yan
College of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorYu-Ting Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Sheng-Hu Yan
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCheng Guo
College of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorYue Zhang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Jia-Yin Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hang-Dong Zuo
School of Safety Science and Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorHua-Feng Yan
College of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorYu-Ting Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Sheng-Hu Yan
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCheng Guo
College of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorYue Zhang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Jia-Yin Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
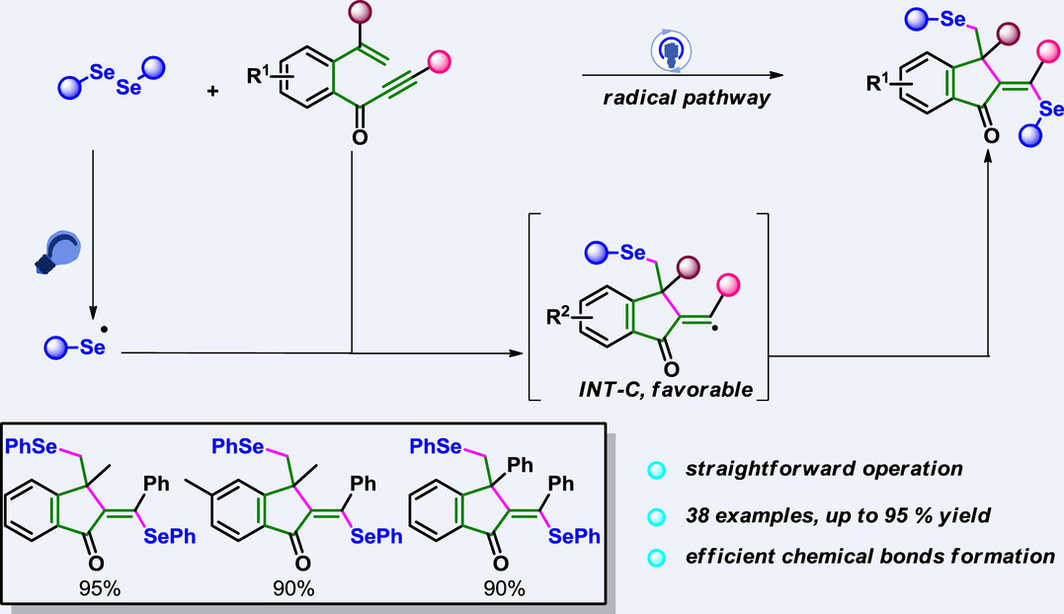
A practical photocatalytic annulation-biselenylation strategy has been developed for the efficient synthesis of biselenium-substituted 1-indanones (38 examples in total) with generally good yields (up to 95%) and excellent stereoselectivity (>19 : 1 Z/E ratio) by employing enynones and diaryl selenides as starting materials under photosensitizer-free conditions. The reaction mechanism involves a cascade process comprising homolytic cleavage, radical addition, 5-exo-dig cyclization, and radical capture, enabling sequential formation of multiple bonds, such as C(sp3)-Se, C(sp3)-C(sp2), and C(sp2)-Se bonds, to rapidly construct molecular complexity. Notably, this approach demonstrates wide substrate compatibility and excellent tolerability towards various functional groups. It is further characterized by its remarkable efficiency in creating chemical bonds and achieving high atomic utilization of 100%.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500079-sup-0001-supinfo.pdfPDF document, 15.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Zhang, Z.; Gevorgyan, V. Visible Light-Induced Reactions of Diazo Compounds and Their Precursors. Chem. Rev. 2024, 124, 7214–7261; (b) Xie, Z.-Y.; Xuan, J. Advances in heterocycle synthesis through photochemical carbene transfer reactions. Chem. Commun. 2024, 60, 2125–2136; (c) Dutta, S.; Erchinger, J. E.; Strieth-Kalthoff, F.; Kleinmans, R.; Glorius, F. Energy transfer photocatalysis: exciting modes of reactivity. Chem. Soc. Rev. 2024, 53, 1068–1089; (d) Wortman, A. K.; Stephenson, C. R. J. EDA photochemistry: Mechanisticinvestigations and future opportunities. Chem 2023, 9, 2390–2415; (e) Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Photocatalytic Late-Stage C–H Functionalization. Chem. Rev. 2023, 123, 4237–4352; (f) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C-C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561.
- 2For selected examples, see: (a) Boyle, B. T.; Dow, N. W.; Kelly, C. B.; Bryan, M. C.; MacMillan, D. W. C. Unlocking carbene reactivity by metallaphotoredox α-elimination. Nature 2024, 631, 789–795; (b) Chan, A. Y.; Ghosh, A.; Yarranton, J. T.; Twilton, J.; Jin, J.; Arias-Rotondo, D. M.; Sakai, H. A.; McCusker, J. K.; MacMillan, D. W. C. Exploiting the Marcus Inverted Region for First-Row Transition Metal−Based Photoredox Catalysis. Science 2023, 382, 191–197; (c) Pipal, R. W.; Stout, K. T.; Musacchio, P. Z.; Ren, S.; Graham, T. J. A.; Verhoog, S.; Gantert, L.; Lohith, T. G.; Schmitz, A.; Lee, H. S.; Hesk, D.; Hostetler, E. D.; Davies, I. W.; MacMillan, D. W. C. Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery. Nature 2021, 589, 542–547; (d) Dong, Z.; MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 2021, 598, 451–456.
- 3For selected examples, see: (a) Chu, J. C. K.; Rovis, T. Amide-directed photoredoxcatalysed C–C bond formation at unactivated sp3 C–H bonds. Nature 2016, 539, 272–275; (b) Ravetz, B. D.; Pun, A. B.; Churchill, E. M.; Congreve, D. N.; Rovis, T.; Campos, L. M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346; (c) Xie, K. A.; Bednarova, E.; Joe, C. L.; Sherwood, T. C.; Welin, E. R.; Rovis, T. A Unified Method for Oxidative and Reductive Decarboxylative Arylation with Orange Light-Driven Ir/Ni Metallaphotoredox Catalysis. J. Am. Chem. Soc. 2024, 146, 25780–25787; (d) Zhang, X.; Shen, Y.; Rovis, T. Photoinduced Nickel-Catalyzed Selective N-Demethylation of Trialkylamines Using C(sp2)-Bromides as HAT Reagents. J. Am. Chem. Soc. 2023, 145, 3294–3300.
- 4For selected examples, see: (a) Harmata, A. S.; Tatunashvili, E.; Chang, A.; Wang, T.; Stephenson, C. R. J. Bicyclo[2.1.1]hexanes via Intramolecular Formal (3+2)-Cycloaddition. Angew. Chem. Int. Ed. 2025, 64, e202413695; (b) Wortman, A. K.; Stephenson, C. R. J. EDA photochemistry: Mechanistic investigations and future opportunities. Chem 2023, 9, 2390–2415; (c) Sun, A. C.; Steyer, D. J.; Robinson, R. I.; Ginsburg-Moraff, C.; Plummer, S.; Gao, J.; Tucker, J. W.; Alpers, D.; Stephenson, C. R. J.; Kennedy, R. T. High-Throughput Optimization of Photochemical Reactions Using Segmented-Flow Nanoelectrospray- Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2023, 62, e202301664; (d) Harmata, A. S.; Spiller, T. E.; Sowden, M. J.; Stephenson, C. R. J. Photochemical Formal (4 + 2)-Cycloaddition of ImineSubstituted Bicyclo[1.1.1]pentanes and Alkenes. J. Am. Chem. Soc. 2021, 143, 21223–21228.
- 5For selected examples, see: (a) Cai, Y.; Roy, T. K.; Zähringer, T. J. B.; Lansbergen, B.; Kerzig, C.; Ritter, T. Arylthianthrenium Salts for Triplet Energy Transfer Catalysis. J. Am. Chem. Soc. 2024, 146, 30474–30482; (b) Ni, S.; Halder, R.; Ahmadli, D.; Reijerse, E. J.; Cornella, J.; Ritter, T. C-heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat. Catal. 2024, 7, 733–741; (c) Kim, J.; Sun, X.; van der Worp, B. A.; Ritter, T. AntiMarkovnikov Hydrochlorination and Hydronitrooxylation of α-Olefins via Visible-light Photocatalysis. Nat. Catal. 2023, 6, 196–203; (d) Su, W.; Xu, P.; Ritter, T. Decarboxylative Hydroxylation of Benzoic Acids. Angew. Chem. Int. Ed. 2021, 60, 24012–24017.
- 6For selected examples, see: (a) Martynova, E. A.; Voloshkin, V. V.; Villa, M.; Fiorentino, A.; Beliš, M.; Hecke, K. V.; Ceroni, P.; Nolan, S. P. Access to cyclobutene-fused dihydrobenzothiophenes via gold-mediated photocatalyzed [2+2]-cycloaddition reactions. J. Catal. 2025, 442, 115850; (b) Qin, H.-N.; Jiang, H.-W.; Zhao, Y.; Qurban, S.; Wang, K.-C.; Xu, P.-F. Photocatalytic [3 + 2]-annulation via sodium tetraarylborate: a fundamental approach for synthesizing 1,4,2-diazaborole analogs. Chem. Sci. 2025, 16, 2837–2842; (c) Lin, S.-L.; Zhao, F.; Wei, F.; Shi, Y.-T.; Wen, J.-K.; Yang, C.; Zhang, H.-S.; Li, C.-C.; Liu, C.; Ye, W.-C.; Cheng, M.-J.; Wang, L. Visible-Light Photocatalyzed Skeletal Rearrangement Enables the Synthesis of Highly Functionalized Xanthenes with Antitumor Activity. Angew. Chem. Int. Ed. 2025, e202420671; (d) Yin, Y.; Chen, F.; Chen, D.; Xie, P.; Wang, D.; Loh, T.-P. Iron-Photocatalyzed Decarboxylative Alkylation of Carboxylic Acids with Morita-Baylis-Hillman Acetates. Org. Lett. 2025, 27, 269–274; (e) Zhang, K.; Gao, Z.; Xia, Y.; Li, P.; Gao, P.; Duan, X.-H.; Guo, L.-N. Synthesis of fluorine-containing bicyclo[4.1.1]octenes via photocatalyzed defluorinative (4 + 3) annulation of bicyclo[1.1.0]butanes with gem-difluoroalkenes. Chem. Sci. 2025, 16, 1411–1416; (f) Zhang, B.; Li, T.-T.; Mao, Z.-C.; Jiang, M.; Zhang, Z.; Zhao, K.; Qu, W.-Y.; Xiao, W.-J.; Chen, J.-R. Enantioselective Cyanofunctionalization of Aromatic Alkenes via Radical Anions. J. Am. Chem. Soc. 2024, 146, 1410–1422; (g) Yang, D.; Yan, Q.; Zhu, E.; Lv, J.; He, W.-M. Carbon-sulfur bond formation via photochemical strategies: An efficient method for the synthesis of sulfur-containing compounds. Chin. Chem. Lett. 2022, 33, 1798–1816; (h) Meng, X.-X.; Kang, Q.-Q.; Zhang, J.-Y.; Li, Q.; Wei, W.-T.; He, W.-M. Visiblelight-initiated regioselective sulfonylation/cyclization of 1,6-enynes under photocatalyst- and additive-free conditions. Green Chem. 2020, 22, 1388–1392; (i) Hu, J.; Yuan, X.; Li, Y.; Chen, X.; Nie, Z.; Chiou, M.-F.; Li, Y.; Bao, H. Photocatalyzed Dual Strain Release of [1.1.1]Propellane with Diazo Compounds. ACS Catal. 2024, 14, 5481–5490; (j) Huang, Y.-L.; Zhang, Q.-Q.; Wang, C.-Y.; Zhao, Y.; Wang, X.-S. Development of SF6 as a Formal Electrophilic Fluorinating Reagent for Photocatalyzed Oxidative Fluorination of Phosphine Oxides. Org. Lett. 2024, 26, 5776–5781; (k) Zuo, H.-D.; Chen, X.; Yuan, Y.-Y.; Zhang, Y.; Liu, J.-W.; Yan, S.-H.; Hao, W.-J.; Jiang, B. Photocatalytic Bicyclization of Indole-Tethered 1,6-Enynes for Diastereoselective Synthesis of Pyrrolo[3,2,1-jk]carbazoles. Org. Lett. 2024, 26, 3810–3815; (l) Zuo, H.-D.; Chen, X.; Zhang, Y.; Liu, J.-W.; Yan, S.-H.; Li, G.; Wang, J.-Y. Photocatalytic Thio/Selenosulfonylation–Bicyclization of Indole-Tethered 1,6-Enynes Leading to Substituted Benzo[c]pyrrolo[1,2,3-lm]carbazoles. Org. Lett. 2024, 26, 3828–3833.
- 7For selected reviews, see: (a) Cheung, K. P. S.; Sarkar, S.; Gevorgyan, V. Visible Light-Induced Transition Metal Catalysis. Chem. Rev. 2022, 122, 1543–1625; (b) Zhang, J.; Rueping, M. Metallaphotoredox catalysis for sp3 C–H functionalizations through hydrogen atom transfer (HAT). Chem. Soc. Rev. 2023, 52, 4099–4120; (c) de Groot, L. H. M.; Ilic, A.; Schwarz, J.; Wärnmark, K. Iron Photoredox Catalysis-Past, Present, and Future. J. Am. Chem. Soc. 2023, 145, 9369–9388; (d) Beaudelot, J.; Oger, S.; Peruško, S.; Phan, T.-A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609; (e) Chan, A. Y.; Perry, I. B.; Bissonnette, N. B.; Buksh, B. F.; Edwards, G. A.; Frye, L. I.; Garry, O. L.; Lavagnino, M. N.; Li, B. X.; Liang, Y.; Mao, E.; Millet, A.; Oakley, J. V.; Reed, N. L.; Sakai, H. A.; Seath, C. P.; MacMillan, D. W. C. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542; (f) Cabanero, D. C.; Rovis, T. Low-energy photoredox catalysis. Nat. Rev. Chem. 2025, 9, 28–45.
- 8(a) Kim, S.-H.; Kwon, S.-H.; Park, S.-H.; Lee, J.-K.; Bang, H.-S.; Nam, S.-J.; Oh, D.-C.; Kwon, H. C.; Shin, J. Tripartin, a histone demethylase inhibitor from a bacterium associated with a dung beetle larva. Org. Lett. 2013, 15, 1834–1837; (b) DeSolms, S. J.; Woltersdorf, O. W., Jr; Cragoe, E. J., Jr; Watson, L. S.; Fanelli, G. M., Jr (Acylaryloxy)acetic acid diuretics. 2. (2-Alkyl-2-aryl-1-oxo-5-indanyloxy)acetic acids. J. Med. Chem. 1978, 21, 437–443; (c) Wessig, P.; Teubner, J. Total Synthesis of Pterosines B and C via a Photochemical Key Step. Synlett 2006, 10, 1543–1546; (d) Yao, W.; Wasserman, Z. R.; Chao, M.; Reddy, G.; Shi, E.; Liu, R. Q.; Covington, M. B.; Arner, E. C.; Pratta, M. A.; Tortorella, M.; Magolda, R. L.; Newton, R.; Qian, M.; Ribadeneira, M. D.; Christ, D.; Wexler, R. R.; Decicco, C. P. Design and synthesis of a series of (2R)-N4-hydroxy-2-(3-hydroxybenzyl)-N1-[(1S,2R)-2-hydroxy- 2,3-dihydro-1H-inden-1-yl] butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J. Med. Chem. 2001, 44, 3347–3350; (e) Ito, T.; Tanaka, T.; Iinuma, M.; Nakaya, K.-i.; Takahashi, Y.; Sawa, R.; Murata, J.; Darnaedi, D. Three New Resveratrol Oligomers from the Stem Bark of Vatica pauciflora. J. Nat. Prod. 2004, 67, 932–937.
- 9(a) Fillion, E.; Fishlock, D.; Wilsily, A.; Goll, J. M. Meldrum's Acids as Acylating Agents in the Catalytic Intramolecular Friedel-Crafts reaction. J. Org. Chem. 2005, 70, 1316–1327; (b) Chassaing, S.; Kumarraja, M.; Pale, P.; Sommer, J. Zeolite-Directed Cascade Reactions: Cycliacyarylation versus Decarboxyarylation of α,β-Unsaturated Carboxylic Acids. Org. Lett. 2007, 9, 3889–3892; (c) Oliverio, M.; Nardi, M.; Costanzo, P.; Cariati, L.; Cravotto, G.; Giofre, S. V.; Procopio, A. Non-Conventional Methodologies in the Synthesis of 1-Indanones. Molecules 2014, 19, 5599–5610; (d) Lu, W.-X.; Mao, J.-G.; Xing, J.; Tang, H.-Y.; Liao, J.; Quan, Y.-s.; Lu, Z.-M.; Yang, Z.-J.; Shen, C. Palladium-Catalyzed Synthesis of Indanone via C–H Annulation Reaction of Aldehydes with Norbornenes. J. Org. Chem. 2024, 89, 784–792; (e) Xiao, Y.; Huang, D.-W.; Liao, J.; Wang, B.; Li, Y.-L.; Wang, J.-Y. Fe(III)-Catalyzed Ring Expansion of Cyclopropenone from Olefins via Radicals to Access Pyrone and Indanone Derivatives. Org. Lett. 2025, 27, 814–820; (f) Sar, S.; Ghorai, P. Pd-Catalyzed Chemodivergent Synthesis of 1-Indanones via Diethyl Zinc-Mediated Precise Switching of Umpolung Reactivity. ACS Catal. 2023, 13, 9706–9712; (g) Ano, Y.; Takahashi, D.; Yamada, Y.; Chatani, N. Palladium-Catalyzed Skeletal Rearrangement of Cyclobutanones via C–H and C-C Bond Cleavage. ACS Catal. 2023, 13, 2234–2239; (h) Chen, L.; Shi, C.; Li, W.; Li, B.; Zhu, J.; Lin, A.; Yao, H. Palladium-Catalyzed Asymmetric C–C Bond Activation/Carbonylation of Cyclobutanones. Org. Lett. 2022, 24, 9157–9162; (i) Zhao, W.; Montgomery, J. Functionalization of Styrenes by Copper-Catalyzed Borylation/ ortho-Cyanation and Silver-Catalyzed Annulation Processes. Angew. Chem. Int. Ed. 2015, 54, 12683–12686; (j) Gagnier, S. V.; Larock, R. C. Palladium-Catalyzed Carbonylative Cyclization of Unsaturated Aryl Iodides and Dienyl Triflates, Iodides, and Bromides to Indanones and 2-Cyclopentenones. J. Am. Chem. Soc. 2003, 125, 4804–4807; (k) Cai, S.-L.; Li, Y.; Yang, C.; Sheng, J.; Wang, X.-S. NHC Ligand Enabled, Palladium-Catalyzed Non-Directed C(sp3)–H Carbonylation to Access Indanone Cores. ACS Catal. 2019, 9, 10299–10304; (l) Xie, Y.; Bao, Y.-P.; Zhuo, X.-Y.; Xuan, J. Photocatalytic Synthesis of Indanone, Pyrone, and PyridinoneDerivatives with Diazo Compounds as Radical Precursors. Org. Lett. 2024, 26, 1393–1398; (m) Shi, C.; Liu, R.; Wang, Z.; Li, X.; Qin, H.; Yuan, L.; Shan, W.; Zhuang, W.; Li, X.; Shi, D. Radical Addition-Enabled C-C σ-Bond Cleavage/Reconstruction to Access Functional Indanones: Total Synthesis of Carexane L. Org. Lett. 2024, 26, 2913–2917; (n) Mao, Y.; Fan, P.; Wang, C. Photocatalyzed Formal All-Carbon [3+2] Cycloaddition of Aromatic Aldehydes with Arylethynyl Silanes. Org. Lett. 2022, 24, 9413–9418; (o) Zhang, T.-S.; Hao, W.-J.; Wang, R.; Wang, S.-C.; Tu, S.-J.; Jiang, B. Electrocatalytic three-component annulationhalosulfonylation of 1,6-enynes toward 1-indanones using sodium halides as both halogen sources and electrolytes. Green Chem. 2020, 22, 4259–4269.
- 10(a) Wang, J.-Y.; Zhang, S.; Tang, Y.; Yan, S.; Li, G. Copper-Catalyzed Annulation-Trifluoromethyl Functionalization of Enynones. Org. Lett. 2023, 25, 2509–2514; (b) Wang, J.-Y.; Zhang, S.; Yuan, Q.; Li, G.; Yan, S. Catalytic Radical-Triggered Annulation/Iododifluoromethylation of Enynones for the Stereospecific Synthesis of 1-Indenones. J. Org. Chem. 2023, 88, 8532–8541; (c) Yan, H.-F.; Zou, X.; Wang, J.-Q.; Guo, C.; Zuo, H.-D. Metal-free radical cascade cyclization/haloazidation of enynones to access functionalized 1-indanones. Org. Biomol. Chem. 2025, 23, 1067–1072.
- 11(a) Krakowiak, A.; Pietrasik, S. New Insights into Oxidative and Reductive Stress Responses and Their Relation to the Anticancer Activity of Selenium-Containing Compounds as Hydrogen Selenide Donors. Biology 2023, 12, 875;
(b) Tanini, D.; Capperucci, A. The Chemistry of Selenosilanes: A Topic Overview. Molecules 2024, 29, 4595;
(c) Lenardão, E. J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds, Springer, New York, 2018;
10.1007/978-3-319-92405-2 Google Scholar(d) Panda, S.; Panda, A.; Zade, S. S. Organoselenium compounds as fluorescent probes. Coord. Chem. Rev. 2015, 300, 86–100.
- 12For selected reviews: (a) Xu, Y.; Huang, D.; Wu, J.; Wu, X. Recent Developments in Selenylation of Alkynes. Adv. Synth. Catal. 2024, 366, 3761–3789;
(b) Protti, S.; Fagnoni, M. Recent Advances in Light-Induced Selenylation. ACS Org. Inorg. Au 2022, 2, 455–463;
(c) Nomoto, A.; Higuchi, Y.; Kobiki, Y.; Ogawa, A. Synthesis of selenium compounds by free radical addition based on visible-light-activated Se-Se bond cleavage. Mini-Rev. Med. Chem. 2013, 13, 814–823;
(d) Wang, X.; Zhang, Y.; Sun, K.; Meng, J.; Zhang, B. Study on the Application of Photoelectric Technology in the Synthesis of Selenium-Containing Heterocycles. Chin. J. Org. Chem. 2021, 41, 4588–4609 (in Chinese);
10.6023/cjoc202109046 Google Scholar(e) Cai, J.; Cen, K.; Li, W.; Lin, H.; Zhang, H. Electrochemical Synthesis of 4-Selenylated Oxazolones via Oxyselenylation of Ynamides. Adv. Synth. Catal. 2025, 367, e202401146; (f) Li, B.; Zhou, Y.; Xu, Y.; Li, X.; Li, Z.; Gu, L.; Ma, W.; Mei, R. Transition-Metal-Free Electrochemical Selenylative Cyclization of Alkynyl Phosphonates. J. Org. Chem. 2023, 88, 15414–15427; (g) Fu, Z.; Yin, J.; He, D.; Yi, X.; Guo, S.; Cai, H. An Electrochemical Method for Deborylative Selenylation of Arylboronic Acids under Metal- and Oxidant-free Conditions. Green Chem. 2022, 24, 130–135; (h) Qu, P.; Jiang, Y.-Q.; Wu, H.; Wang, Y.-H.; Ling, Y.; Zhang, Y.; Liu, G.-Q. Photoinduced trans-Diastreoselective Oxyselenenylation of Allylic Alcohols to Form Selenylated Cyclic Boronic Esters. Adv. Synth. Catal. 2024, 366, 3160–3165; (i) Zhang, S.; Yuan, J.; Huang, G.; Ma, C.; Yang, J.; Yang, L.; Xiao, Y.; Qu, L. Visible-Light-Induced Intramolecular Tandem Cyclization of Unactivated Indoloalkynes for the Synthesis of Sulfonylated and Selenylated Indolo[1,2-a]quinolines. J. Org. Chem. 2023, 88, 11712–11727; (j) Patil, D. V.; Hong, Y. T.; Kim, H. Y.; Oh, K. Visible-Light-Induced Three-Component Selenofunctionalization of Alkenes: An Aerobic Selenol Oxidation Approach. Org. Lett. 2022, 24, 8465–8469; (k) Wan, Y.; Li, C.; Lin, Z.; Lin, X.; Gao, H.; Yi, W.; Zhou, Z. Assembly of Selenadiazine Scaffolds via Rh(III)-Catalyzed Amidine- Directed Cascade C–H Selenylation/[5 + 1] Annulation with Elemental Selenium. Org. Lett. 2024, 26, 6625–6630; (l) Bai, X.; Chen, J.; Du, H.; Zhao, C.; Li, Y.; Li, Y.; Dixneuf, P. H.; Zhang, M.; Chen, L. Silver-Mediated Acetoxyselenylation of Alkynes: Mild Stereoselective Access to Bifunctional Alkenes. Org. Lett. 2024, 26, 9811–9816; (m) Badshah, G.; Gomes, C. M. B.; Ali, S.; Luz, E. Q.; Silvério, G. L.; Santana, F. S.; Seckler, D.; Paixão, D. B.; Schneider, P. H.; Rampon, D. S. Palladium- Catalyzed Direct Selanylation of Chalcogenophenes and Arenes Assisted by 2-(Methylthio)Amide. J. Org. Chem. 2023, 88, 14033–14047; (n) Liu, L.; Jian, Y.; Hu, W.; Zhao, S.; Shi, Z.-J.; Selander, N.; Zhou, T. Ni and Fe catalyzed cascade radical reactions of oxime esters with diselenides. Org. Chem. Front. 2022, 9, 3480–3485.
- 13(a) Xu, Z.; Yao, J.; Zhong, K.; Lin, S.; Hu, X.; Ruan, Z. Electrochemical Selenylation of Sulfoxonium Ylides for the Synthesis of gem-Diselenides as Antimicrobials against Fungi. J. Org. Chem. 2023, 88, 5572–5585; (b) Zhu, J.; Ye, Y.; Yan, Y.; Sun, J.; Huang, Y. Highly Regioselective Dichalcogenation of Alkenyl Sulfonium Salts to Access 1,1-Dichalcogenalkenes. Org. Lett. 2023, 25, 5324–5328; (c) Zhou, P.; Jiao, H.; Niu, K.; Song, H.; Liu, Y.; Wang, Q. Facile and General Electrochemical Diselenylation of Terminal Alkynes. ACS Sustainable Chem. Eng. 2023, 11, 2607–2612; (d) Roy, M.; Jamatia, R.; Samanta, A.; Mohar, K.; Srimani, D. Change in the Product Selectivity in the Visible Light-Induced Selenium Radical-Mediated 1,4-Aryl Migration Process. Org. Lett. 2022, 24, 8180–8185; (e) Chen, H.; Ding, R.; Tang, H.; Pan, Y.; Xu, Y.; Chen, Y. Simultaneous construction of C–Se and C–S bonds via the visible-light mediated multicomponent cascade reaction of diselenides, alkynes, and SO2. Chem. Asian J. 2019, 14, 3264–3268; (f) Zhang, Z.; Wang, S.; Tan, P.; Gu, X.; Sun, W.; Liu, C.; Chen, J.; Li, J.; Sun, K. K2S2O8/I2-Promoted Electrophilic Selenylative Cyclization to Access Seleno-Benzo[b]azepines. Org. Lett. 2022, 24, 2288–2293; (g) Wang, Z.; Wang, X.; Li, Q.; Ni, S.; Zhao, D.; Yang, S.; Qiu, G.; Sun, K. Chemoselective Electrochemical Seleno-Cyclization of Dienes to Medium-Sized Benzo[b]azocines. Chin. Chem. Lett. 2023, 35, 109058.
- 14(a) Ogawa, A.; Obayashi, R.; Doi, M.; Sonoda, N.; Hirao, T. A Novel Photoinduced Thioselenation of Allenes by Use of a Disulfide-Diselenide Binary System. J. Org. Chem. 1998, 63, 4277–4281; (b) Ogawa, A.; Ogawa, I.; Obayashi, R.; Umezu, K.; Doi, M.; Hirao, T. Highly Selective Thioselenation of Vinylcyclopropanes with a (PhS)2-(PhSe)2 Binary System and Its Application to Thiotelluration. J. Org. Chem. 1999, 64, 86–92; (c) Yamamoto, Y.; Tanaka, R.; Ota, M.; Nishimura, M.; Tran, C. C.; Kawaguchi, S.-i.; Kodama, S.; Nomoto, A.; Ogawa, A. Photoinduced Syntheses and Reactivities of Phosphorus-Containing Interelement Compounds. J. Org. Chem. 2020, 85, 14708–14719; (d) Ji, X.-S.; Zuo, H.-D.; Shen, Y.-T.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Electrochemical selective annulative amino-ketalization and amino-oxygenation of 1,6-enynes. Chem. Commun. 2022, 58, 10420–10423; (e) Yuan, Y.-Y.; Chen, X.; Wang, J.-Y.; Yan, S.-H.; Wang, Y.-T.; Zhang, Y.; Liu, J.-W.; Zuo, H.-D. Electrocatalytic AnnulationIodosulfonylation of Indole-Tethered 1,6-Enynes to Access Pyrrolo-[1,2-a]indoles. Eur. J. Org. Chem. 2024, 27, e202301256; (f) Zuo, H.-D.; Yuan, Y.-Y.; Chen, X.; Wang, J.-Y.; Yan, S.-H.; Zhang, Y.; Liu, J.-W. Copper-Catalyzed Radical-Induced Annulation-Halo(bi)cyanomethylation of Indole-Tethered 1,6-Enynes toward Pyrrolo[1,2-a]indoles. Adv. Synth. Catal. 2024, 366, 3578–3584.




