Recent Natural Product Total Syntheses Involving Cycloadditions of Allenes
Chen-Chen Gu
Department of Chemistry, Guangming Advanced Research Institute, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorXiong-En Long
School of Pharmacy and Food Engineering, Wuyi University, Jiangmen, Guangdong, 529020 China
Search for more papers by this authorXiuping Chen
State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China
Search for more papers by this authorCorresponding Author
Junyang Liu
School of Pharmacy and Food Engineering, Wuyi University, Jiangmen, Guangdong, 529020 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuang-Chuang Li
Department of Chemistry, Guangming Advanced Research Institute, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorChen-Chen Gu
Department of Chemistry, Guangming Advanced Research Institute, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorXiong-En Long
School of Pharmacy and Food Engineering, Wuyi University, Jiangmen, Guangdong, 529020 China
Search for more papers by this authorXiuping Chen
State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China
Search for more papers by this authorCorresponding Author
Junyang Liu
School of Pharmacy and Food Engineering, Wuyi University, Jiangmen, Guangdong, 529020 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuang-Chuang Li
Department of Chemistry, Guangming Advanced Research Institute, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorAbstract
Comprehensive Summary
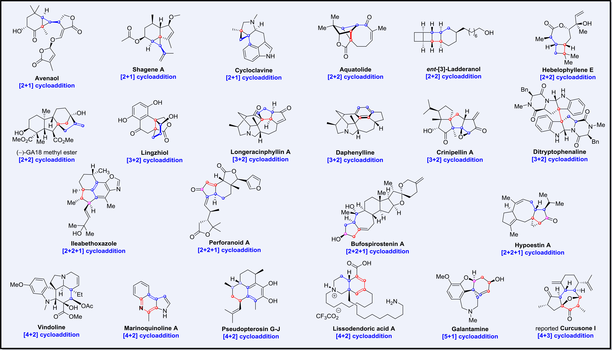
Allenes, characterized by their cumulated carbon–carbon double bonds, have emerged as indispensable synthons in the construction of complex natural products. Their unique reactivity and stereochemical properties render allenes a powerful tool for the efficient and streamlined total synthesis of structurally intricate natural products. This review comprehensively summarizes the total syntheses of complex natural products involving allene cycloaddition reactions reported over the past decade (2014—2024). Among the nearly 20 total syntheses reviewed, the [2+1], [2+2], [3+2], [4+2], [4+3], [5+1] and [2+2+1] cycloaddition reactions of allenes are the most important transformations to construct the key skeleton. This is because of their ability to form multiple bonds in a single step with high atom economy, stereoselectivity, and regioselectivity, often under mild conditions. The strategic application of these reactions in forming key carbon–carbon bonds and controlling stereochemistry makes them practical for the efficient assembly of complex molecular frameworks. With the ongoing exploration of methods for allene generation, particularly the enantioselective approaches, and the continued discovery of novel cyclization reactions of allenes, allene chemistry will maintain its crucial and indispensable role in the total synthesis of natural products.
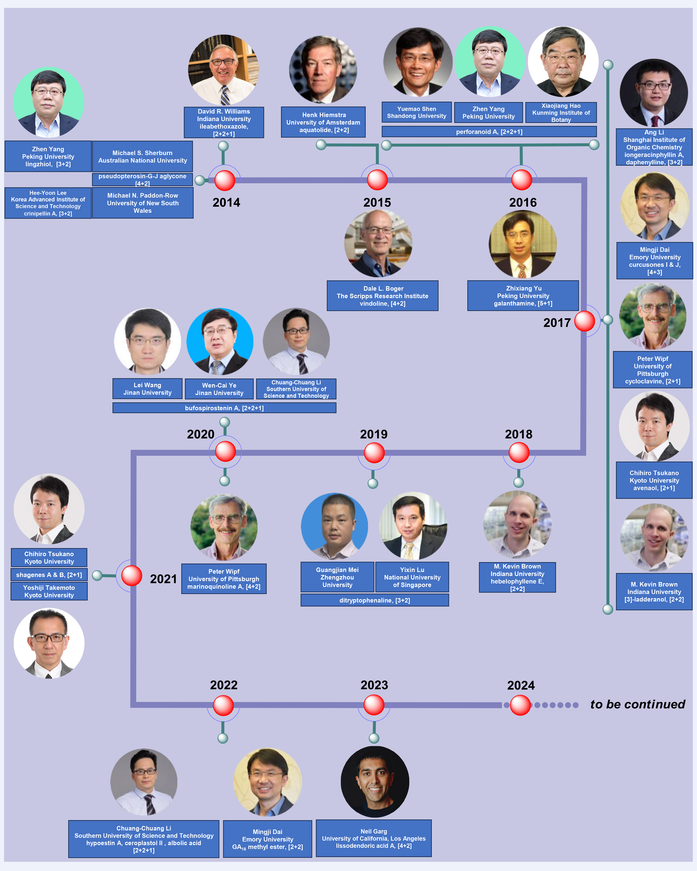
Key Scientists
References
- 1 Zimmer, R.; Dinesh, C. U.; Nandanan, E.; Khan, F. A. Palladium-Catalyzed Reactions of Allenes. Chem. Rev. 2000, 100, 3067–3126.
- 2 Taylor, D. R. The chemistry of allenes. Chem. Rev. 1967, 67, 317–359.
- 3 Pasto, D. J. Recent developments in allene chemistry. Tetrahedron 1984, 40, 2805–2827.
- 4 Alcaide, B.; Almendros, P. Progress in allene chemistry. Chem. Soc. Rev. 2014, 43, 2886–2887.
- 5 Krause, N.; Hashmi, A. S. K. Modern Allene Chemistry, WILEY-VCH Verlag GmbH & Co. KGaA, 2004.
- 6 Grissom, J. W.; Gunawardena, G. U.; Klingberg, D.; Huang, D. The chemistry of enediynes, enyne allenes and related compounds. Tetrahedron 1996, 52, 6453–6518.
- 7 Ye, J.; Ma, S. Palladium-Catalyzed Cyclization Reactions of Allenes in the Presence of Unsaturated Carbon–Carbon Bonds. Acc. Chem. Res. 2014, 47, 989–1000.
- 8 López, F.; Mascareñas, J. L. Allenes as Three-Carbon Units in Catalytic Cycloadditions: New Opportunities with Transition-Metal Catalysts. Chem. - Eur. J. 2011, 17, 418–428.
- 9 Chu, W.-D.; Zhang, Y.; Wang, J. Recent advances in catalytic asymmetric synthesis of allenes. Catal. Sci. Technol. 2017, 7, 4570–4579.
- 10 Fu, L.; Greßies, S.; Chen, P.; Liu, G. Recent Advances and Perspectives in Transition Metal-Catalyzed 1,4-Functionalizations of Unactivated 1,3-Enynes for the Synthesis of Allenes. Chin. J. Chem. 2020, 38, 91–100.
- 11 Li, Y.; Bao, H. Radical transformations for allene synthesis. Chem. Sci. 2022, 13, 8491–8506.
- 12 Yu, S.; Ma, S. How easy are the syntheses of allenes? Chem. Commun. 2011, 47, 5384–5418.
- 13 Brummond, K. M.; DeForrest, J. E. Synthesizing Allenes Today (1982–2006). Synthesis 2007, 2007, 795–818.
- 14 Ye, J.; Ma, S. Conquering three-carbon axial chirality of allenes. Org. Chem. Front. 2014, 1, 1210–1224.
- 15 Singh, J.; Saxena, B.; Sharma, A. Visible light promoted synthesis of allenes. Catal. Sci. Technol. 2024, 14, 5143–5160.
- 16 Hoffmann-Röder, A.; Krause, N. Synthesis and Properties of Allenic Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2004, 43, 1196–1216.
- 17 Yu, S.; Ma, S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112.
- 18 Ma, S. Some Typical Advances in the Synthetic Applications of Allenes. Chem. Rev. 2005, 105, 2829–2872.
- 19 Gampe, C. M.; Carreira, E. M. Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem. Int. Ed. 2012, 51, 3766–3778.
- 20 Brandi, A.; Goti, A. Synthesis of Methylene- and Alkylidenecyclopropane Derivatives. Chem. Rev. 1998, 98, 589–636.
- 21 Alcaide, B.; Almendros, P.; Aragoncillo, C. Exploiting [2+2] cycloaddition chemistry: achievements with allenes. Chem. Soc. Rev. 2010, 39, 783–816.
- 22 Alcaide, B.; Almendros, P. The Allenic Pauson−Khand Reaction in Synthesis. Eur. J. Org. Chem. 2004, 2004, 3377–3383.
- 23 Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Engaging Yne-Allenes in Cycloaddition Reactions: Recent Developments. Chin. J. Chem. 2022, 40, 1224–1242.
- 24 Wang, Z.; Xu, X.; Kwon, O. Phosphine catalysis of allenes with electrophiles. Chem. Soc. Rev. 2014, 43, 2927–2940.
- 25 Sahu, S. K.; Behera, P. K.; Choudhury, P.; Sethi, M.; Jena, S.; Rout, L. Recent advances in [3+2] cycloaddition of allenes with 1,3-carbonyl ylides; Rh(ii)-catalyzed access to bridged polyoxocarbocyles. New J. Chem. 2021, 45, 11018–11029.
- 26 Sikandar, S.; Zahoor, A. F.; Ghaffar, A.; Anjum, M. N.; Noreen, R.; Irfan, A.; Munir, B.; Kotwica-Mojzych, K.; Mojzych, M. Unveiling the Chemistry and Synthetic Potential of Catalytic Cycloaddition Reaction of Allenes: A Review. Molecules 2023, 28, 704.
- 27 Cardoso, A. L.; Soares, M. I. 1,3-Dipolar cycloadditions involving allenes: synthesis of five-membered rings. Curr. Org. Chem. 2019, 23, 3064–3134.
- 28
Pinho e Melo, T. M. Allenes as dipolarophiles and 1,3-dipole precursors: Synthesis of carbocyclic and heterocyclic compounds. Curr. Org. Chem. 2009, 13, 1406–1431.
10.2174/138527209789055090 Google Scholar
- 29 López, F.; Mascareñas, J. L. [4+2] and [4+3] catalytic cycloadditions of allenes. Chem. Soc. Rev. 2014, 43, 2904–2915.
- 30 Kitagaki, S.; Inagaki, F.; Mukai, C. [2+2+1] Cyclization of allenes. Chem. Soc. Rev. 2014, 43, 2956–2978.
- 31 Qiu, Y.; Zhou, J.; Fu, C.; Ma, S. A General Diversified Synthesis of Carbazoles and the First Synthesis of Karapinchamine A. Chem. - Eur. J. 2014, 20, 14589–14593.
- 32 Rao, N. N.; Cha, J. K. Concise Synthesis of Alkaloid (−)-205B. J. Am. Chem. Soc. 2015, 137, 2243–2246.
- 33 Magné, V.; Lorton, C.; Marinetti, A.; Guinchard, X.; Voituriez, A. Short Enantioselective Total Synthesis of (−)-Rhazinilam Using a Gold(I)- Catalyzed Cyclization. Org. Lett. 2017, 19, 4794–4797.
- 34 Yang, Z.; Tan, Q.; Jiang, Y.; Yang, J.; Su, X.; Qiao, Z.; Zhou, W.; He, L.; Qiu, H.; Zhang, M. Asymmetric Total Synthesis of Sarpagine and Koumine Alkaloids. Angew. Chem. Int. Ed. 2021, 60, 13105–13111.
- 35 Zhou, W.; Xi, S.; Chen, H.; Jiang, D.; Yang, J.; Liu, S.; He, L.; Qiu, H.; Lan, Y.; Zhang, M. A bridged backbone strategy enables collective synthesis of strychnan alkaloids. Nat. Chem. 2023, 15, 1074–1082.
- 36 Ji, Y.; Liu, Y.; Guan, W.; Guo, C.; Jia, H.; Hong, B.; Li, H. Enantioselective Divergent Syntheses of Diterpenoid Pyrones. J. Am. Chem. Soc. 2024, 146, 9395–9403.
- 37 Nishiyama, T.; Kihara, Y.; Takeuchi, N.; Mizuno, S.; Hieda, Y.; Hatae, N.; Choshi, T. Total syntheses of carbazole alkaloid mukoenine A and pyrano[3,2-a]carbazole alkaloid girinimbine. Tetrahedron 2022, 120, 132895.
- 38 Hieda, Y.; Une, Y.; Satoh, A.; Tsuruga, T.; Choshi, T. Total synthesis of the reported structure of bioactive dibenzofuran natural product karnatakafuran B. Tetrahedron 2023, 135, 133327.
- 39 Liu, W.; Wang, B. Synthesis of (±)-Merrilactone A by a Desymmetrization Strategy. Chem. - Eur. J. 2018, 24, 16511–16515.
- 40 Xu, B.; Xun, W.; Su, S.; Zhai, H. Total Syntheses of (−)-Conidiogenone B, (−)-Conidiogenone, and (−)-Conidiogenol. Angew. Chem. Int. Ed. 2020, 59, 16475–16479.
- 41 Lv, N.; Han, J.-C.; Zhang, P.; Huang, Y.-R.; Xu, Z.-X.; Xu, K.; Wang, X.-F.; Li, X.; Chung, L. W.; Li, C.-C. Intramolecular [3 + 2] annulation of allenylsilane-enes: Direct synthesis of highly strained trans-fused 5/5 ring systems. Chem 2024, 10, 190–198.
- 42 Li, L.-Z.; Huang, Y.-R.; Xu, Z.-X.; He, H.-S.; Ran, H.-W.; Zhu, K.-Y.; Han, J.-C.; Li, C.-C. Synthesis of Bridged Five-Membered Ring Systems by Type II [3 + 2] Annulation of Allenylsilane-ene. J. Am. Chem. Soc. 2024, 146, 24782–24787.
- 43 Huo, C.-Y.; Zheng, T.-L.; Dai, W.-H.; Zhang, Z.-H.; Wang, J.-D.; Zhu, D.-Y.; Wang, S.-H.; Zhang, X.-M.; Xu, X.-T. InI3-catalyzed polyene cyclization of allenes and its application in the total synthesis of seven abietane-type diterpenoids. Chem. Sci. 2022, 13, 13893–13897.
- 44 Zhao, L.-P.; Li, P.-J.; Wang, L.; Tang, Y. Allenamide-Initiated Cascade [2+2+2] Annulation Enabling the Divergent Total Synthesis of (−)-Deoxoapodine, (−)-Kopsifoline D and (±)-Melotenine A. Angew. Chem. Int. Ed. 2022, 61, e202207360.
- 45Nakano, S.-i.; Hamada, Y.; Nemoto, T. Enantioselective formal synthesis of (−)-aurantioclavine using Pd-catalyzed cascade cyclization and organocatalytic asymmetric aziridination. Tetrahedron Lett. 2018, 59, 760–762.
- 46 Kim, H. I.; Kisugi, T.; Khetkam, P.; Xie, X.; Yoneyama, K.; Uchida, K.; Yokota, T.; Nomura, T.; McErlean, C. S. P.; Yoneyama, K. Avenaol, a germination stimulant for root parasitic plants from Avena strigosa. Phytochemistry 2014, 103, 85–88.
- 47 Yasui, M.; Ota, R.; Tsukano, C.; Takemoto, Y. Total synthesis of avenaol. Nat. Commun. 2017, 8, 674.
- 48 Goddard-Borger, E. D.; Stick, R. V. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org. Lett. 2007, 9, 3797–3800.
- 49 Lindsay, V. N. G.; Fiset, D.; Gritsch, P. J.; Azzi, S.; Charette, A. B. Stereoselective Rh2(S-IBAZ)4-Catalyzed Cyclopropanation of Alkenes, Alkynes, and Allenes: Asymmetric Synthesis of Diacceptor Cyclopropylphosphonates and Alkylidenecyclopropanes. J. Am. Chem. Soc. 2013, 135, 1463–1470.
- 50 Li, H.; Mazet, C. Catalyst-Directed Diastereoselective Isomerization of Allylic Alcohols for the Stereoselective Construction of C(20) in Steroid Side Chains: Scope and Topological Diversification. J. Am. Chem. Soc. 2015, 137, 10720–10727.
- 51 Mantilli, L.; Mazet, C. Iridium-catalyzed isomerization of primary allylic alcohols under mild reaction conditions. Tetrahedron Lett. 2009, 50, 4141–4144.
- 52 Wüstenberg, B.; Pfaltz, A. Homogeneous Hydrogenation of Tri- and Tetrasubstituted Olefins: Comparison of Iridium-Phospinooxazoline [Ir-PHOX] Complexes and Crabtree Catalysts with Hexafluorophosphate (PF6) and Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BArF) as Counterions. Adv. Synth. Catal. 2008, 350, 174–178.
- 53 Curci, R.; D'Accolti, L.; Fiorentino, M.; Fusco, C.; Adam, W.; González-Nu~nez, M. E.; Mello, R. Oxidation of acetals, an orthoester, and ethers by dioxiranes through α-CH insertion. Tetrahedron Lett. 1992, 33, 4225–4228.
- 54 von Salm, J. L.; Wilson, N. G.; Vesely, B. A.; Kyle, D. E.; Cuce, J.; Baker, B. J. Shagenes A and B, New Tricyclic Sesquiterpenes Produced by an Undescribed Antarctic Octocoral. Org. Lett. 2014, 16, 2630–2633.
- 55 Tsukano, C.; Yagita, R.; Heike, T.; Mohammed, T. A.; Nishibayashi, K.; Irie, K.; Takemoto, Y. Asymmetric Total Synthesis of Shagenes A and B. Angew. Chem. Int. Ed. 2021, 60, 23106–23111.
- 56 Ostermeier, M.; Brunner, B.; Korff, C.; Helmchen, G. Highly Enantioselective Rhodium-Catalyzed Hydrogenation of 2-(2-Methoxy-2-oxoethyl)acrylic Acid − A Convenient Access of Enantiomerically Pure Isoprenoid Building Blocks. Eur. J. Org. Chem. 2003, 2003, 3453–3459.
- 57 Murphy, S. K.; Dong, V. M. Enantioselective Ketone Hydroacylation Using Noyori's Transfer Hydrogenation Catalyst. J. Am. Chem. Soc. 2013, 135, 5553–5556.
- 58 Bolte, B.; Odabachian, Y.; Gagosz, F. Gold(I)-Catalyzed Rearrangement of Propargyl Benzyl Ethers: A Practical Method for the Generation and in Situ Transformation of Substituted Allenes. J. Am. Chem. Soc. 2010, 132, 7294–7296.
- 59 Espino, C. G.; Fiori, K. W.; Kim, M.; Du Bois, J. Expanding the Scope of C−H Amination through Catalyst Design. J. Am. Chem. Soc. 2004, 126, 15378–15379.
- 60 Stauffacher, D.; Niklaus, P.; Tscherter, H.; Weber, H. P.; Hofmann, A. Cycloclavin, ein neues alkaloid aus Ipomoea hildebrandtii vatke—71: Mutterkornalkaloide. Tetrahedron 1969, 25, 5879–5887.
- 61 Furuta, T.; Koike, M.; Abe, M. Isolation of Cycloclavine from the Culture Broth of Aspergillus japonicus SAITO. Agric. Biol. Chem. 1982, 46, 1921–1922.
- 62 McCabe, S. R.; Wipf, P. Eight-Step Enantioselective Total Synthesis of (−)-Cycloclavine. Angew. Chem. Int. Ed. 2017, 56, 324–327.
- 63 Goto, T.; Takeda, K.; Anada, M.; Ando, K.; Hashimoto, S. Enantio- and diastereoselective cyclopropanation with tert-butyl α-diazopropionate catalyzed by dirhodium(II) tetrakis[N-tetrabromophthaloyl-(S)- tert-leucinate]. Tetrahedron Lett. 2011, 52, 4200–4203.
- 64 Heiner, T.; Kozhushkov, S. I.; Noltemeyer, M.; Haumann, T.; Boese, R.; de Meijere, A. Intramolecular Diels-Alder reactions of furans with a merely strain-activated tetrasubstituted alkene: Bicyclopropylidene. Tetrahedron 1996, 52, 12185–12196.
- 65 Petronijevic, F.; Timmons, C.; Cuzzupe, A.; Wipf, P. A microwave assisted intramolecular-furan-Diels–Alder approach to 4-substituted indoles. Chem. Commun. 2008, 106.
- 66 A. Padwa; Flick, A. C. Chapter One - Intramolecular Diels–Alder Cycloaddition of Furans (IMDAF) for Natural Product Synthesis. In Advances in Heterocyclic Chemistry, Vol. 110, Ed.: A. R. Katritzky, Academic Press, 2013, pp. 1–41.
- 67 Boonsompat, J.; Padwa, A. An IMDAF Cycloaddition Approach toward the Synthesis of the Lycopodium Alkaloid (±)-Fawcettidine. J. Org. Chem. 2011, 76, 2753–2761.
- 68 LaPorte, M.; Hong, K. B.; Xu, J.; Wipf, P. 5-Hydroxyindoles by Intramolecular Alkynol–Furan Diels–Alder Cycloaddition. J. Org. Chem. 2013, 78, 167–174.
- 69 DeAngelis, A.; Dmitrenko, O.; Fox, J. M. Rh-Catalyzed Intermolecular Reactions of Cyclic α-Diazocarbonyl Compounds with Selectivity over Tertiary C–H Bond Migration. J. Am. Chem. Soc. 2012, 134, 11035–11043.
- 70 DeAngelis, A.; Dmitrenko, O.; Yap, G. P. A.; Fox, J. M. Chiral Crown Conformation of Rh2(S-PTTL)4: Enantioselective Cyclopropanation with α-Alkyl-α-diazoesters. J. Am. Chem. Soc. 2009, 131, 7230–7231.
- 71 Diao, T.; Stahl, S. S. Synthesis of Cyclic Enones via Direct Palladium- Catalyzed Aerobic Dehydrogenation of Ketones. J. Am. Chem. Soc. 2011, 133, 14566–14569.
- 72 Stahl, S. S.; Diao, T. 7.06 Oxidation Adjacent to CX Bonds by Dehydrogenation. In Comprehensive Organic Synthesis, 2nd ed., Ed.: P. Knochel, Elsevier, 2014, pp. 178–212.
- 73
Henry-Riyad, H.; Tidwell, T. T. Thermolysis of N-tetramethylpiperidinyl esters: homolytic fragmentation and induced decomposition. ARKIVOC 2008, 2008, 113–126.
10.3998/ark.5550190.0009.a10 Google Scholar
- 74 McCabe, S. R.; Wipf, P. Asymmetric Total Synthesis and Biological Evaluation of (+)-Cycloclavine. Synthesis 2019, 51, 213–224.
- 75 San Feliciano, A.; Medarde, M.; Miguel del Corral, J. M.; Aramburu, A.; Gordaliza, M.; Barrero, A. F. Aquatolide. A new type of humulane-related sesquiterpene lactone. Tetrahedron Lett. 1989, 30, 2851–2854.
- 76 Lodewyk, M. W.; Soldi, C.; Jones, P. B.; Olmstead, M. M.; Rita, J.; Shaw, J. T.; Tantillo, D. J. The Correct Structure of Aquatolide— Experimental Validation of a Theoretically-Predicted Structural Revision. J. Am. Chem. Soc. 2012, 134, 18550–18553.
- 77 Saya, J. M.; Vos, K.; Kleinnijenhuis, R. A.; van Maarseveen, J. H.; Ingemann, S.; Hiemstra, H. Total Synthesis of Aquatolide. Org. Lett. 2015, 17, 3892–3894.
- 78 Crabbé, P.; Fillion, H.; André, D.; Luche, J.-L. Efficient homologation of acetylenes to allenes. J. Chem. Soc., Chem. Commun. 1979, 859–860.
- 79 Kuang, J.; Ma, S. An Efficient Synthesis of Terminal Allenes from Terminal 1-Alkynes. J. Org. Chem. 2009, 74, 1763–1765.
- 80 Cockerill, G. S.; Kocienski, P.; Treadgold, R. Silicon-mediated annulation. Part 1. A synthesis of tetrahydropyran-4-ones, oxepan-4-ones, and oxocan-4-ones via intramolecular directed aldol reactions. J. Chem. Soc., Perkin Trans. 1 1985, 2093–2100.
- 81 Sinninghe Damsté, J. S.; Strous, M.; Rijpstra, W. I. C.; Hopmans, E. C.; Geenevasen, J. A. J.; van Duin, A. C. T.; van Niftrik, L. A.; Jetten, M. S. M. Linearly concatenated cyclobutane lipids form a dense bacterial membrane. Nature 2002, 419, 708–712.
- 82 Sinninghe Damsté, J. S.; Rijpstra, W. I. C.; Geenevasen, J. A. J.; Strous, M.; Jetten, M. S. M. Structural identification of ladderane and other membrane lipids of planctomycetes capable of anaerobic ammonium oxidation (anammox). FEBS J. 2005, 272, 4270–4283.
- 83 Novak, I. Ring Strain in [n]ladderanes. J. Phys. Chem. A 2008, 112, 10059–10063.
- 84 Raghavan, V.; Johnson, J. L.; Stec, D. F.; Song, B.; Zajac, G.; Baranska, M.; Harris, C. M.; Schley, N. D.; Polavarapu, P. L.; Harris, T. M. Absolute Configurations of Naturally Occurring [5]- and [3]-Ladderanoic Acids: Isolation, Chiroptical Spectroscopy, and Crystallography. J. Nat. Prod. 2018, 81, 2654–2666.
- 85 Boumann, H. A.; Hopmans, E. C.; Van De Leemput, I.; Op den Camp, H. J. M.; Van De Vossenberg, J.; Strous, M.; Jetten, M. S. M.; Sinninghe Damsté, J. S.; Schouten, S. Ladderane phospholipids in anammox bacteria comprise phosphocholine and phosphoethanolamine headgroups. FEMS Microbiol. Lett. 2006, 258, 297–304.
- 86 Line, N. J.; Witherspoon, B. P.; Hancock, E. N.; Brown, M. K. Synthesis of ent-[3]-Ladderanol: Development and Application of Intramolecular Chirality Transfer [2+2] Cycloadditions of Allenic Ketones and Alkenes. J. Am. Chem. Soc. 2017, 139, 14392–14395.
- 87 Guo, R.; Brown, M. K. Lewis Acid-Promoted [2 + 2] Cycloadditions of Allenes and Ketenes: Versatile Methods for Natural Product Synthesis. Acc. Chem. Res. 2023, 56, 2253–2264.
- 88 Guo, R.; Beattie, S. R.; Krysan, D. J.; Brown, M. K. Enantioselective Synthesis of (+)-Hippolide J and Reevaluation of Antifungal Activity. Org. Lett. 2020, 22, 7743–7746.
- 89 Guo, R.; Witherspoon, B. P.; Brown, M. K. Evolution of a Strategy for the Enantioselective Synthesis of (−)-Cajanusine. J. Am. Chem. Soc. 2020, 142, 5002–5006.
- 90 Liu, H.; Leow, D.; Huang, K.-W.; Tan, C.-H. Enantioselective Synthesis of Chiral Allenoates by Guanidine-Catalyzed Isomerization of 3-Alkynoates. J. Am. Chem. Soc. 2009, 131, 7212–7213.
- 91 Inokuma, T.; Furukawa, M.; Uno, T.; Suzuki, Y.; Yoshida, K.; Yano, Y.; Matsuzaki, K.; Takemoto, Y. Bifunctional Hydrogen-Bond Donors That Bear a Quinazoline or Benzothiadiazine Skeleton for Asymmetric Organocatalysis. Chem. - Eur. J. 2011, 17, 10470–10477.
- 92 Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts. Org. Lett. 2005, 7, 1967–1969.
- 93 Stegbauer, L.; Sladojevich, F.; Dixon, D. J. Bifunctional organo/metal cooperative catalysis with cinchona alkaloid scaffolds. Chem. Sci. 2012, 3, 942–958.
- 94 Zhao, J.-F.; Loh, T.-P. Acid-Catalyzed Intramolecular [2+2] Cycloaddition of Ene-allenones: Facile Access to Bicyclo[n.2.0] Frameworks. Angew. Chem. Int. Ed. 2009, 48, 7232–7235.
- 95 Ondet, P.; Lemière, G.; Duñach, E. Cyclisations Catalysed by Bismuth(III) Triflate. Eur. J. Org. Chem. 2017, 2017, 761–780.
- 96 Mascitti, V.; Corey, E. J. Enantioselective Synthesis of Pentacycloanammoxic Acid. J. Am. Chem. Soc. 2006, 128, 3118–3119.
- 97 Kirmse, W. 100 Years of the Wolff Rearrangement. Eur. J. Org. Chem. 2002, 2002, 2193–2256.
- 98 Wichlacz, M.; Ayer, W. A.; Trifonov, L. S.; Chakravarty, P.; Khasa, D. cis-Fused caryophyllenes from liquid cultures of Hebeloma longicaudum. Phytochemistry 1999, 51, 873–877.
- 99 Wichlacz, M.; Ayer, W. A.; Trifonov, L. S.; Chakravarty, P.; Khasa, D. Two 6,7-seco-caryophyllenes and an alloaromadendrane from liquid cultures of Hebeloma longicaudum. Phytochemistry 1999, 52, 1421–1425.
- 100 Wichlacz, M.; Ayer, W. A.; Trifonov, L. S.; Chakravarty, P.; Khasa, D. A Caryophyllene-Related Sesquiterpene and Two 6,7-Seco-caryophyllenes from Liquid Cultures of Hebeloma longicaudum. J. Nat. Prod. 1999, 62, 484–486.
- 101 Wahl, J. M.; Conner, M. L.; Brown, M. K. Synthesis of (−)-Hebelophyllene E: An Entry to Geminal Dimethyl-Cyclobutanes by [2+2] Cycloaddition of Alkenes and Allenoates Synthesis of (−)-Hebelophyllene E: An Entry to Geminal Dimethyl-Cyclobutanes by [2+2] Cycloaddition of Alkenes and Allenoates. Angew. Chem. Int. Ed. 2018, 57, 4647–4651.
- 102
Anh, N. T.; Eisenstein, O. Induction asymetrique 1–2: comparaison ab initio des modeles de cram, de cornforth, de karabatsos et de felkin. Tetrahedron Lett. 1976, 17, 155–158.
10.1016/0040-4039(76)80002-0 Google Scholar
- 103
Nguyen Trong, A.; Eisenstein, O.; Lefour, J. M.; Tran Huu Dau, M. E. Orbital factors and asymmetric induction. J. Am. Chem. Soc. 1973, 95, 6146–6147.
10.1021/ja00799a068 Google Scholar
- 104 Conner, M. L.; Xu, Y.; Brown, M. K. Catalytic Enantioselective Allenoate–Alkene [2 + 2] Cycloadditions. J. Am. Chem. Soc. 2015, 137, 3482–3485.
- 105 Kang, T.; Ge, S.; Lin, L.; Lu, Y.; Liu, X.; Feng, X. A Chiral N,N′-Dioxide–ZnII Complex Catalyzes the Enantioselective [2+2] Cycloaddition of Alkynones with Cyclic Enol Silyl Ethers. Angew. Chem. Int. Ed. 2016, 55, 5541–5544.
- 106 Hu, J.-L.; Feng, L.-W.; Wang, L.; Xie, Z.; Tang, Y.; Li, X. Enantioselective Construction of Cyclobutanes: A New and Concise Approach to the Total Synthesis of (+)-Piperarborenine B. J. Am. Chem. Soc. 2016, 138, 13151–13154.
- 107 Lipshutz, B. H.; Servesko, J. M. CuH-Catalyzed Asymmetric Conjugate Reductions of Acyclic Enones. Angew. Chem. Int. Ed. 2003, 42, 4789–4792.
- 108 Lipshutz, B. H.; Servesko, J. M.; Taft, B. R. Asymmetric 1,4-Hydrosilylations of α,β-Unsaturated Esters. J. Am. Chem. Soc. 2004, 126, 8352–8353.
- 109 Lipshutz, B. H.; Tanaka, N.; Taft, B. R.; Lee, C.-T. Chiral Silanes via Asymmetric Hydrosilylation with Catalytic CuH. Org. Lett. 2006, 8, 1963–1966.
- 110 MacMillan, J. Occurrence of Gibberellins in Vascular Plants, Fungi, and Bacteria. J. Plant Growth Regul. 2001, 20, 387–442.
- 111
Koshimizu, K.; Fukui, H.; Kusaki, T.; Mitsui, T.; Ogawa, Y. A new C20 gibberellin in immature seeds of lupinus luteus. Tetrahedron Lett. 1966, 7, 2459–2463.
10.1016/S0040-4039(00)75676-0 Google Scholar
- 112 Koshimizu, K.; Fukui, H.; Kusaki, T.; Ogawa, Y.; Mitsui, T. Isolation and Structure of Gibberellin A18 from Immature Seeds of Lupinus luteus. Agric. Biol. Chem. 1968, 32, 1135–1140.
- 113 Li, L.; Liang, W.; Rivera, M. E.; Wang, Y.-C.; Dai, M. Concise Synthesis of (−)-GA18 Methyl Ester. J. Am. Chem. Soc. 2023, 145, 53–57.
- 114 Takatori, K.; Ota, S.; Tendo, K.; Matsunaga, K.; Nagasawa, K.; Watanabe, S.; Kishida, A.; Kogen, H.; Nagaoka, H. Synthesis of Methylenebicyclo[3.2.1]octanol by a Sm(II)-Induced 1,2-Rearrangement Reaction with Ring Expansion of Methylenebicyclo[4.2.0]octanone. Org. Lett. 2017, 19, 3763–3766.
- 115 Yan, Y.-M.; Ai, J.; Zhou, L. L.; Chung, A. C. K.; Li, R.; Nie, J.; Fang, P.; Wang, X.-L.; Luo, J.; Hu, Q.; et al. Lingzhiols, Unprecedented Rotary Door-Shaped Meroterpenoids as Potent and Selective Inhibitors of p-Smad3 from Ganoderma lucidum. Org. Lett. 2013, 15, 5488–5491.
- 116 Long, R.; Huang, J.; Shao, W.; Liu, S.; Lan, Y.; Gong, J.; Yang, Z. Asymmetric total synthesis of (−)-lingzhiol via a Rh-catalysed [3+2] cycloaddition. Nat. Commun. 2014, 5, 5707.
- 117 Justik, M. W.; Koser, G. F. Oxidative rearrangements of arylalkenes with [hydroxy(tosyloxy)iodo]benzene in 95% methanol: a general, regiospecific synthesis of α-aryl ketones. Tetrahedron Lett. 2004, 45, 6159–6163.
- 118 Corey, E. J.; Bakshi, R. K.; Shibata, S. Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987, 109, 5551–5553.
- 119
Conia, J. M.; Le Perchec, P. The Thermal Cyclisation of Unsaturated Carbonyl Compounds. Synthesis 1975, 1975, 1–19.
10.1055/s-1975-23652 Google Scholar
- 120 Zheng, N.; Zhang, L.; Gong, J.; Yang, Z. Formal Total Synthesis of (±)-Lycojaponicumin C. Org. Lett. 2017, 19, 2921–2924.
- 121 Shao, W.; Huang, J.; Guo, K.; Gong, J.; Yang, Z. Total Synthesis of Sinensilactam A. Org. Lett. 2018, 20, 1857–1860.
- 122 J. Zhong; H. Wang; Q. Zhang; Gao, S. Chapter Two - The chemistry of Daphniphyllum alkaloids. In The Alkaloids: Chemistry and Biology, Vol. 85, Ed.: H.-J. Knölker, Academic Press, 2021, pp. 113–176.
- 123 Di, Y.-T.; He, H.-P.; Lu, Y.; Yi, P.; Li, L.; Wu, L.; Hao, X.-J. Alkaloids from the Leaves of Daphniphyllum longeracemosum. J. Nat. Prod. 2006, 69, 1074–1076.
- 124 Li, J.; Zhang, W.; Zhang, F.; Chen, Y.; Li, A. Total Synthesis of Longeracinphyllin A. J. Am. Chem. Soc. 2017, 139, 14893–14896.
- 125 Zhang, C.; Lu, X. Phosphine-Catalyzed Cycloaddition of 2,3-Butadienoates or 2-Butynoates with Electron-Deficient Olefins. A Novel [3 + 2] Annulation Approach to Cyclopentenes. J. Org. Chem. 1995, 60, 2906–2908.
- 126 Xu, Z.; Lu, X. Phosphine-catalyzed [3+2] cycloaddition reactions of substituted 2-alkynoates or 2,3-allenoates with electron-deficient olefins and imines. Tetrahedron Lett. 1999, 40, 549–552.
- 127 Lu, X.; Zhang, C.; Xu, Z. Reactions of Electron-Deficient Alkynes and Allenes under Phosphine Catalysis. Acc. Chem. Res. 2001, 34, 535–544.
- 128 Liang, Y.; Liu, S.; Xia, Y.; Li, Y.; Yu, Z.-X. Mechanism, Regioselectivity, and the Kinetics of Phosphine-Catalyzed [3+2] Cycloaddition Reactions of Allenoates and Electron-Deficient Alkenes. Chem. - Eur. J. 2008, 14, 4361–4373.
- 129 Xia, Y.; Liang, Y.; Chen, Y.; Wang, M.; Jiao, L.; Huang, F.; Liu, S.; Li, Y.; Yu, Z.-X. An Unexpected Role of a Trace Amount of Water in Catalyzing Proton Transfer in Phosphine-Catalyzed (3 + 2) Cycloaddi–tion of Allenoates and Alkenes. J. Am. Chem. Soc. 2007, 129, 3470–3471.
- 130 Wallace, D. J.; Sidda, R. L.; Reamer, R. A. Phosphine-Catalyzed Cycloadditions of Allenic Ketones: New Substrates for Nucleophilic Catalysis. J. Org. Chem. 2007, 72, 1051–1054.
- 131 Zhang, Q.; Di, Y.-T.; Li, C.-S.; Fang, X.; Tan, C.-J.; Zhang, Z.; Zhang, Y.; He, H.-P.; Li, S.-L.; Hao, X.-J. Daphenylline, a New Alkaloid with an Unusual Skeleton, from Daphniphyllum longeracemosum. Org. Lett. 2009, 11, 2357–2359.
- 132 Lu, Z.; Li, Y.; Deng, J.; Li, A. Total synthesis of the Daphniphyllum alkaloid daphenylline. Nat. Chem. 2013, 5, 679–684.
- 133 Chen, Y.; Zhang, W.; Ren, L.; Li, J.; Li, A. Total Syntheses of Daphe–nylline, Daphnipaxianine A, and Himalenine D. Angew. Chem. Int. Ed. 2018, 57, 952–956.
- 134 Kupka, J.; Anke, T.; Oberwinkler, F.; Schramm, G.; Steglich, W. Antibiotics from Basidiomycetes. Vii1 Crinipellin, a new antibiotic from the basidiomycetous fungus Crinipellis stipitaria (Fr.) Pat. J. Antibiot. 1979, 32, 130–135.
- 135 Kang, T.; Song, S. B.; Kim, W.-Y.; Kim, B. G.; Lee, H.-Y. Total Synthesis of (−)-Crinipellin A. J. Am. Chem. Soc. 2014, 136, 10274–10276.
- 136 Ohira, S. Methanolysis of Dimethyl (1-Diazo-2-oxopropyl) Phospho–nate: Generation of Dimethyl (Diazomethyl) Phosphonate and Reac–tion with Carbonyl Compounds. Synth. Commun. 1989, 19, 561–564.
- 137 Müller, S.; Liepold, B.; Roth, G. J.; Bestmann, H. J. An Improved One- pot Procedure for the Synthesis of Alkynes from Aldehydes. Synlett 1996, 1996, 521–522.
- 138 Fürstner, A.; Méndez, M. Iron-Catalyzed Cross-Coupling Reactions: Efficient Synthesis of 2,3-Allenol Derivatives. Angew. Chem. Int. Ed. 2003, 42, 5355–5357.
- 139 Kaufman, G. M.; Smith, J. A.; Stouw, G. G. V.; Shechter, H. Pyrolysis of Salts of p-Tosylhydrazones. Simple Methods for Preparing Diazo Compounds and Effecting Their Carbenic Decomposition. J. Am. Chem. Soc. 1965, 87, 935–937.
- 140 Lee, H.-Y.; Kim, Y. Triquinanes from Linear Alkylidene Carbenes via Trimethylenemethane Diyls. J. Am. Chem. Soc. 2003, 125, 10156–10157.
- 141 Lee, H.-Y.; Kim, W.-Y.; Lee, S. Triquinanes from linear ketones via trimethylenemethane diyls. Tetrahedron Lett. 2007, 48, 1407–1410.
- 142 Parish, E. J.; Wei, T.-Y. Allylic Oxidation of Δ5-Steroids with Pyridi–nium Chlorochromate (PCC) and Pyridinium Dichromate (PDC). Synth. Commun. 1987, 17, 1227–1233.
- 143 Johnson, C. R.; Zeller, J. R. N,S-Dimethyl-s-phenylsulfoximine: A reagent for the optical resolution of ketones. Tetrahedron 1984, 40, 1225–1233.
- 144 Schreiber, J.; Maag, H.; Hashimoto, N.; Eschenmoser, A. Dimethyl(methylene)ammonium Iodide. Angew. Chem. Int. Ed. 1971, 10, 330–331.
- 145 Lee, H.; Kang, T.; Lee, H.-Y. Total Synthesis of (±)-Waihoensene. Angew. Chem. Int. Ed. 2017, 56, 8254–8257.
- 146
Springer, J. P.; Bűchi, G.; Kobbe, B.; Demain, A. L.; Clardy, J. The structure of ditryptophenaline - a new metabolite of aspergillus flavus. Tetrahedron Lett. 1977, 18, 2403–2406.
10.1016/S0040-4039(01)83777-1 Google Scholar
- 147 Chan, W.-L.; Tang, X.; Zhang, F.; Quek, G.; Mei, G.-J.; Lu, Y. Phosphine-Catalyzed (3+2) Annulation of Isoindigos with Allenes: Enantioselective Formation of Two Vicinal Quaternary Stereogenic Centers. Angew. Chem. Int. Ed. 2019, 58, 6260–6264.
- 148 Overman, L. E.; Paone, D. V. Enantioselective Total Syntheses of Ditryptophenaline and ent-WIN 64821. J. Am. Chem. Soc. 2001, 123, 9465–9467.
- 149 Song, K.-M.; Park, S.-W.; Hong, W.-H.; Lee, H.; Kwak, S.-S.; Liu, J.-R. Isolation of Vindoline from Catharanthus roseus by Supercritical Fluid Extraction. Biotechnol. Prog. 1992, 8, 583–586.
- 150 Sears, J. E.; Barker, T. J.; Boger, D. L. Total Synthesis of (−)-Vindoline and (+)-4-epi-Vindoline Based on a 1,3,4-Oxadiazole Tandem Intra–molecular [4 + 2]/[3 + 2] Cycloaddition Cascade Initiated by an Allene Dienophile. Org. Lett. 2015, 17, 5460–5463.
- 151 Kanjana-opas, A.; Panphon, S.; Fun, H.-K.; Chantrapromma, S. 4-Methyl-3H-pyrrolo[2,3-c]quinoline. Acta crystallogr., E 2006, 62, o2728–o2730.
- 152 Osano, M.; Jhaveri, D. P.; Wipf, P. Formation of 6-Azaindoles by Intramolecular Diels–Alder Reaction of Oxazoles and Total Synthesis of Marinoquinoline A. Org. Lett. 2020, 22, 2215–2219.
- 153 Suárez, A.; Fu, G. C. A Straightforward and Mild Synthesis of Functionalized 3-Alkynoates. Angew. Chem. Int. Ed. 2004, 43, 3580–3582.
- 154 Shen, H.-C.; Tang, J.-M.; Chang, H.-K.; Yang, C.-W.; Liu, R.-S. Short and Efficient Synthesis of Coronene Derivatives via Ruthenium-Catalyzed Benzannulation Protocol. J. Org. Chem. 2005, 70, 10113–10116.
- 155 Look, S. A.; Fenical, W.; Jacobs, R. S.; Clardy, J. The pseudopterosins: anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc. Natl. Acad. Sci. U. S. A. 1986, 83, 6238–6240.
- 156 Look, S. A.; Fenical, W.; Matsumoto, G. K.; Clardy, J. The pseudopterosins: a new class of antiinflammatory and analgesic diterpene pentosides from the marine sea whip Pseudopterogorgia elisabethae (Octocorallia). J. Org. Chem. 1986, 51, 5140–5145.
- 157 Berrué, F.; McCulloch, M. W. B.; Kerr, R. G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719.
- 158 Newton, C. G.; Drew, S. L.; Lawrence, A. L.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. Pseudopterosin synthesis from a chiral cross- conjugated hydrocarbon through a series of cycloadditions. Nat. Chem. 2015, 7, 82–86.
- 159 Matsumura, K.; Hashiguchi, S.; Ikariya, T.; Noyori, R. Asymmetric Transfer Hydrogenation of α, β-Acetylenic Ketones. J. Am. Chem. Soc. 1997, 119, 8738–8739.
- 160 Tsuji, J.; Ohno, K. Organic syntheses by means of noble metal compounds XXI. Decarbonylation of aldehydes using rhodium complex. Tetrahedron Lett. 1965, 6, 3969–3971.
- 161 Lyakhova, E. G.; Kolesnikova, S. A.; Kalinovsky, A. I.; Berdyshev, D. V.; Pislyagin, E. A.; Kuzmich, A. S.; Popov, R. S.; Dmitrenok, P. S.; Makarieva, T. N.; Stonik, V. A. Lissodendoric Acids A and B, Manzamine-Related Alkaloids from the Far Eastern Sponge Lissodendoryx florida. Org. Lett. 2017, 19, 5320–5323.
- 162 Ippoliti, F. M.; Adamson, N. J.; Wonilowicz, L. G.; Nasrallah, D. J.; Darzi, E. R.; Donaldson, J. S.; Garg, N. K. Total synthesis of lissodendoric acid A via stereospecific trapping of a strained cyclic allene. Science 2023, 379, 261–265.
- 163 Ippoliti, F. M.; Wonilowicz, L. G.; Adamson, N. J.; Darzi, E. R.; Donaldson, J. S.; Nasrallah, D. J.; Mehta, M. M.; Kelleghan, A. V.; Houk, K.; Garg, N. K. Total Synthesis of Lissodendoric Acid A. Angew. Chem. Int. Ed. 2024, e202406676.
- 164 Tadross, P. M.; Stoltz, B. M. A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev. 2012, 112, 3550–3577.
- 165 Takikawa, H.; Nishii, A.; Sakai, T.; Suzuki, K. Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds. Chem. Soc. Rev. 2018, 47, 8030–8056.
- 166 Chidambaram, N.; Chandrasekaran, S. tert-Butyl hydroperoxide- pyridinium dichromate: a convenient reagent system for allylic and benzylic oxidations. J. Org. Chem. 1987, 52, 5048–5051.
- 167 Das, S.; Li, Y.; Lu, L.-Q.; Junge, K.; Beller, M. A General and Selective Rhodium-Catalyzed Reduction of Amides, N-Acyl Amino Esters, and Dipeptides Using Phenylsilane. Chem. - Eur. J. 2016, 22, 7050–7053.
- 168 Liu, J.-Q.; Yang, Y.-F.; Li, X.-Y.; Liu, E.-Q.; Li, Z.-R.; Zhou, L.; Li, Y.; Qiu, M.-H. Cytotoxicity of naturally occurring rhamnofolane diterpenes from Jatropha curcas. Phytochemistry 2013, 96, 265–272.
- 169 Li, Y.; Dai, M. Total Syntheses of the Reported Structures of Curcusones I and J through Tandem Gold Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11624–11627.
- 170 Li, Y.; Wei, M.; Dai, M. Gold catalysis-facilitated rapid synthesis of the daphnane/tigliane tricyclic core. Tetrahedron 2017, 73, 4172–4177.
- 171 Jiao, L.; Yu, Z.-X. Vinylcyclopropane Derivatives in Transition-Metal- Catalyzed Cycloadditions for the Synthesis of Carbocyclic Compounds. J. Org. Chem. 2013, 78, 6842–6848.
- 172 Fumagalli, G.; Stanton, S.; Bower, J. F. Recent Methodologies That Exploit C–C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev. 2017, 117, 9404–9432.
- 173 Sarel, S. Metal-induced rearrangements and insertions into cyclopropyl olefins. Acc. Chem. Res. 1978, 11, 204–211.
- 174 Taber, D. F.; Kanai, K.; Jiang, Q.; Bui, G. Enantiomerically Pure Cyclohexenones by Fe-Mediated Carbonylation of Alkenyl Cyclopropanes. J. Am. Chem. Soc. 2000, 122, 6807–6808.
- 175 Aumann, R. Reactions of strained carbon-carbon bonds with transition metals. 7. Iron carbonyl complexes from vinylcyclopropane. J. Am. Chem. Soc. 1974, 96, 2631–2632.
- 176 Farley, C. M.; Sasakura, K.; Zhou, Y.-Y.; Kanale, V. V.; Uyeda, C. Catalytic [5 + 1]-Cycloadditions of Vinylcyclopropanes and Vinylidenes. J. Am. Chem. Soc. 2020, 142, 4598–4603.
- 177 Jiang, G.-J.; Fu, X.-F.; Li, Q.; Yu, Z.-X. Rh(I)-Catalyzed [5 + 1] Cycloaddition of Vinylcyclopropanes and CO for the Synthesis of α,β- and β,γ-Cyclohexenones. Org. Lett. 2012, 14, 692–695.
- 178
Fan, X.; Liu, C.-H.; Yu, Z.-X. Rhodium(I)-Catalyzed Cycloadditions Involving Vinylcyclopropanes and Their Derivatives. In Rhodium Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2019, pp. 229–276.
10.1002/9783527811908.ch10 Google Scholar
- 179
Janssen, B.; Schäfer, B. Galantamine. ChemTexts 2017, 3, 7.
10.1007/s40828-017-0043-y Google Scholar
- 180 Cozanitis, D. A. The snowdrop, wellspring of galanthamine. Wiener Medizinische Wochenschrift 2021, 171, 205–213.
- 181 Lilienfeld, S. Galantamine — A Novel Cholinergic Drug with a Unique Dual Mode of Action for the Treatment of Patients with Alzheimer's Disease. CNS Drug Rev. 2002, 8, 159–176.
- 182 Liu, C.-H.; Yu, Z.-X. Rh-catalysed [5 + 1] cycloaddition of allenylcyclopropanes and CO: reaction development and application to the formal synthesis of (−)-galanthamine. Org. Biomol. Chem. 2016, 14, 5945–5950.
- 183 Satcharoen, V.; McLean, N. J.; Kemp, S. C.; Camp, N. P.; Brown, R. C. D. Stereocontrolled Synthesis of (−)-Galanthamine. Org. Lett. 2007, 9, 1867–1869.
- 184 Jørgensen, L.; McKerrall, S. J.; Kuttruff, C. A.; Ungeheuer, F.; Felding, J.; Baran, P. S. 14-Step Synthesis of (+)-Ingenol from (+)-3-Carene. Science 2013, 341, 878–882.
- 185 Bayden, A. S.; Brummond, K. M.; Jordan, K. D. Computational Insight Concerning Catalytic Decision Points of the Transition Metal Catalyzed [2 + 2 + 1] Cyclocarbonylation Reaction of Allenes. Organometallics 2006, 25, 5204–5206.
- 186 Rodríguez, I. I.; Rodríguez, A. D.; Wang, Y.; Franzblau, S. G. Ileabethoxazole: a novel benzoxazole alkaloid with antimycobacterial activity. Tetrahedron Lett. 2006, 47, 3229–3232.
- 187 Williams, D. R.; Shah, A. A. Total Synthesis of (+)-Ileabethoxazole via an Iron-Mediated Pauson–Khand [2 + 2 + 1] Carbocyclization. J. Am. Chem. Soc. 2014, 136, 8829–8836.
- 188 Williams, D. R.; Shah, A. A.; Mazumder, S.; Baik, M.-H. Studies of iron-mediated Pauson–Khand reactions of 1,1-disubstituted-allenylsilanes: mechanistic implications for a reactive three-membered iron metallacycle. Chem. Sci. 2013, 4, 238–247.
- 189 Anderson, A. M.; Blazek, J. M.; Garg, P.; Payne, B. J.; Mohan, R. S. Bismuth(III) oxide perchlorate promoted rearrangement of epoxides to aldehydes and ketones. Tetrahedron Lett. 2000, 41, 1527–1530.
- 190 Lv, C.; Yan, X.; Tu, Q.; Di, Y.; Yuan, C.; Fang, X.; Ben-David, Y.; Xia, L.; Gong, J.; Shen, Y.; et al. Isolation and Asymmetric Total Synthesis of Perforanoid A. Angew. Chem. Int. Ed. 2016, 55, 7539–7543.
- 191 Lv, C.; Tu, Q.; Gong, J.; Hao, X.; Yang, Z. Asymmetric total synthesis of (−)-perforanoid A. Tetrahedron 2017, 73, 3612–3621.
- 192 Evans, M. A.; Morken, J. P. Stereoselective Synthesis of Furans by the Pd-Catalyzed Oshima−Utimoto Reaction. Org. Lett. 2005, 7, 3367–3370.
- 193 Zhao, Y.; Snieckus, V. A Practical in situ Generation of the Schwartz Reagent. Reduction of Tertiary Amides to Aldehydes and Hydrozirconation. Org. Lett. 2014, 16, 390–393.
- 194 Molander, G. A.; Argintaru, O. A. Stereospecific Ni-Catalyzed Cross- Coupling of Potassium Alkenyltrifluoroborates with Alkyl Halides. Org. Lett. 2014, 16, 1904–1907.
- 195 Tian, H.-Y.; Ruan, L.-J.; Yu, T.; Zheng, Q.-F.; Chen, N.-H.; Wu, R.-B.; Zhang, X.-Q.; Wang, L.; Jiang, R.-W.; Ye, W.-C. Bufospirostenin A and Bufogargarizin C, Steroids with Rearranged Skeletons from the Toad Bufo bufo gargarizans. J. Nat. Prod. 2017, 80, 1182–1186.
- 196 Cheng, M.-J.; Zhong, L.-P.; Gu, C.-C.; Zhu, X.-J.; Chen, B.; Liu, J.-S.; Wang, L.; Ye, W.-C.; Li, C.-C. Asymmetric Total Synthesis of Bufospirostenin A. J. Am. Chem. Soc. 2020, 142, 12602–12607.
- 197 Tap, A.; Lecourt, C.; Dhambri, S.; Arnould, M.; Galvani, G.; Nguyen Van Buu, O.; Jouanneau, M.; Férézou, J.-P.; Ardisson, J.; Lannou, M.-I.; et al. Alkoxyallene-ynes: Selective Preparation of Bicyclo[5.3.0] Ring Systems Including a δ-Alkoxy Cyclopentadienone. Chem. - Eur. J. 2016, 22, 4938–4944.
- 198 Kawamura, S.; Chu, H.; Felding, J.; Baran, P. S. Nineteen-step total synthesis of (+)-phorbol. Nature 2016, 532, 90–93.
- 199 Ríos, T.; Gómez, F. Albolic acid, a new sesterterpenic acid isolated from insect wax. Tetrahedron Lett. 1969, 2929–2930.
- 200 Wu, X.-D.; Ding, L.-F.; Li, W.-Y.; Cheng, B.; Lei, T.; Zhou, H.-F.; Zhao, Q.-S. Hypoestins A–D: highly modified fusicoccane diterpenoids with promising Cav3.1 calcium channel inhibitory activity from Hypoestes purpurea. Org. Chem. Front. 2022, 9, 3075–3083.
- 201 Wang, Y.-Q.; Xu, K.; Min, L.; Li, C.-C. Asymmetric Total Syntheses of Hypoestin A, Albolic Acid, and Ceroplastol II. J. Am. Chem. Soc. 2022, 144, 10162–10167.
- 202 Han, A.; Tao, Y.; Reisman, S. E. A 16-step synthesis of the isoryanodane diterpene (+)-perseanol. Nature 2019, 573, 563–567.
- 203
Charette, A. B.; Beauchemin, A. Simmons-Smith Cyclopropanation Reaction. In Organic Reactions, John Wiley & Sons, Inc., 2004, pp. 1–415.
10.1002/0471264180.or058.01 Google Scholar




