Palladium-Catalyzed Oxidative Alkynylation of Allenyl Ketones: Access to 3-Alkynyl Poly-substituted Furans†
Bowen Dou
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
Search for more papers by this authorKang Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jianbo Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorBowen Dou
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
Search for more papers by this authorKang Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jianbo Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing, 100871 China
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Furans bearing alkynyl substituents are highly useful in organic synthesis. However, the methodologies to access these important furan derivatives are rather limited. We herein report an efficient synthesis of alkynylated furan derivatives based on Pd-catalyzed oxidative cross-coupling reaction between allenyl ketones and terminal alkynes. This novel synthesis of alkynylated furans with wide substrate scope is operationally simple and tolerates various functional groups. Mechanistically, the formation of the palladium carbene through cycloisomerization and the subsequent alkynyl migratory insertion are proposed as the key steps in the transformation. The reaction reported in this paper further demonstrates the generality of the carbene-based cross coupling.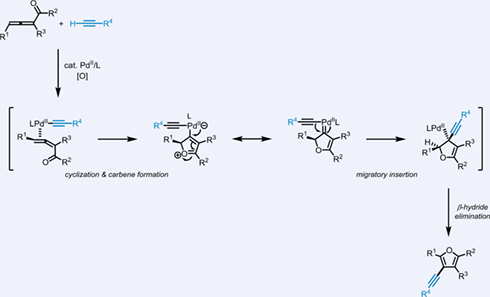
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300465-sup-0001-supinfo.pdfPDF document, 4.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see: (a) Lipshutz, B. H. Five-Membered Heteroaromatic Rings as Intermediates in Organic Synthesis. Chem. Rev. 1986, 86, 795–819; (b) Craig, R. A.; Stoltz, B. M. Polycyclic Furanobutenolide- Derived Cembranoid and Norcembranoid Natural Products: Biosynthetic Connections and Synthetic Efforts. Chem. Rev. 2017, 117, 7878–7909; (c) Gandini, A.; Belgacem, M. N. Furans in Polymer Chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379.
- 2For reviews on furan synthesis, see: (a) Hou, X. L.; Cheung, H. Y.; Hon, T. Y.; Kwan, P. L.; Lo, T. H.; Tong, S. Y. T.; Wong, H. N. C. Regioselective Syntheses of Substituted Furans. Tetrahedron 1998, 54, 1955–2020; (b) Brown, R. C. D. Developments in Furan Syntheses. Angew. Chem. Int. Ed. 2005, 44, 850–852; (c) Kirsch, S. F. Syntheses of Polysubstituted Furans: Recent Developments. Org. Biomol. Chem. 2006, 4, 2076–2080; (d) Patil, N. T.; Yamamoto, Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008, 108, 3395–3442; (e) Godoi, B.; Schumacher, R. F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980; (f) Gulevich, A. V.; Dudnik, A. S.; Chernyak, N.; Gevorgyan, V. Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem. Rev. 2013, 113, 3084–3213; (g) Blanc, A.; Bénéteau, V.; Weibel, J.-M.; Pale, P. Silver & Gold-Catalyzed Routes to Furans and Benzofurans. Org. Biomol. Chem. 2016, 14, 9184–9205; (h) Duc, D. X. Recent Progress in the Synthesis of Furan. Mini-Rev. Org. Chem. 2019, 16, 422–452; (i) Karlinskii, B. Y.; Ananikov, V. P. Catalytic C−H Functionalization of Unreactive Furan Cores in Bio-Derived Platform Chemicals. ChemSusChem 2021, 14, 558–568; (j) Nejrotti, S.; Prandi, C. Gold Catalysis and Furans: A Powerful Match for Synthetic Connections. Synthesis 2021, 53, 1046–1060.
- 3For selected examples, see: (a) Jung, C.-K.; Wang, J.-C.; Krische, M. J. Phosphine-Mediated Reductive Condensation of γ-Acyloxy Butynoates: A Diversity Oriented Strategy for the Construction of Substituted Furans. J. Am. Chem. Soc. 2004, 126, 4118–4119; (b) Yao, T.; Zhang, X.; Larock, R. C. AuCl3-Catalyzed Synthesis of Highly Substituted Furans from 2-(1-Alkynyl)-2-alken-1-ones. J. Am. Chem. Soc. 2004, 126, 11164–11165; (c) Xiao, Y.; Zhang, J. Tetrasubstituted Furans by a PdII-Catalyzed Three-Component Michael Addition/Cyclization/Cross-Coupling Reaction. Angew. Chem. Int. Ed. 2008, 47, 1903–1906; (d) Lenden, P.; Entwistle, D. A.; Willis, M. C. An Alkyne Hydroacylation Route to Highly Substituted Furans. Angew. Chem. Int. Ed. 2011, 50, 10657–10660; (e) He, C.; Guo, S.; Ke, J.; Hao, J.; Xu, H.; Chen, H.; Lei, A. Silver-Mediated Oxidative C–H/C–H Functionalization: A Strategy to Construct Polysubstituted Furans. J. Am. Chem. Soc. 2012, 134, 5766–5769; (f) Cui, X.; Xu, X.; Wojtas, L.; Kim, M. M.; Zhang, X. P. Regioselective Synthesis of Multisubstituted Furans via Metalloradical Cyclization of Alkynes with α-Diazocarbonyls: Construction of Functionalized α-Oligofurans. J. Am. Chem. Soc. 2012, 134, 19981–19984; (g) Lian, Y.; Huber, T.; Hesp, K. D.; Bergman, R. G.; Ellman, J. A. Rhodium(III)-Catalyzed Alkenyl C–H Bond Functionalization: Convergent Synthesis of Furans and Pyrroles. Angew. Chem. Int. Ed. 2013, 52, 629–633; (h) Lu, B.; Wu, J.; Yoshikai, K. Palladium-Catalyzed Condensation of N-Aryl Imines and Alkynylbenziodoxolones to Form Multisubstituted Furans. J. Am. Chem. Soc. 2014, 136, 11598–11601; (i) He, X.; Tang, Y.; Wang, Y.; Chen, J.-B.; Xu, S.; Dou, J.; Li, Y. Phosphine-Catalyzed Activation of Alkylidenecyclopropanes: Rearrangement to Form Polysubstituted Furans and Dienones. Angew. Chem. Int. Ed. 2019, 58, 10698–10702; (j) Chen, V. Y.; Kwon, O. Unified Approach to Furan Natural Products via Phosphine-Palladium Catalysis. Angew. Chem. Int. Ed. 2021, 60, 8874–8881; (k) Li, L.; Kail, S.; Weber, S. M.; Hilt, G. Indium-Catalysed Transfer Hydrogenation for the Reductive Cyclisation of 2-Alkynyl Enones towards Trisubstituted Furans. Angew. Chem. Int. Ed. 2021, 60, 23661–23666; (l) Haut, F. L.; Habiger, C.; Wein, L. A.; Wurst, K.; Podewitz, M.; Magauer, T. Rapid Assembly of Tetrasubstituted Furans via Pummerer-Type Rearrangement. J. Am. Chem. Soc. 2021, 143, 1216–1223.
- 4(a) Marshall, J. A.; Robinson, E. D. A Mild Method for the Synthesis of Furans. Application to 2,5-Bridged Furan Macrocyclic Compounds. J. Org. Chem. 1990, 55, 3450–3451; (b) Marshall, J. A.; Wang, X. J. Synthesis of Furans by Ag(I)-Promoted Cyclization of Allenyl Ketones and Aldehydes. J. Org. Chem. 1991, 56, 960–969; (c) Marshall, J. A.; Bartley, G. S. Observations Regarding the Ag(I)-Catalyzed Conversion of Allenones to Furans. J. Org. Chem. 1994, 59, 7169–7171.
- 5(a) Hashmi, A. S. K. Transition Metal Catalyzed Dimerization of Allenyl Ketones. Angew. Chem. Int. Ed. 1995, 34, 1581–1583;
(b) Hashmi, A. S. K.; Schwarz, L.; Choi, J.-H.; Frost, T. M. A New Gold-Catalyzed C−C Bond Formation. Angew. Chem. Int. Ed. 2000, 39, 2285–2288.
10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 6(a) Sromek, A. W.; Rubina, M.; Gevorgyan, V. 1,2-Halogen Migration in Haloallenyl Ketones: Regiodivergent Synthesis of Halofurans. J. Am. Chem. Soc. 2005, 127, 10500–10501; (b) Dudnik, A. S.; Gevorgyan, V. Metal-Catalyzed [1,2]-Alkyl Shift in Allenyl Ketones: Synthesis of Multisubstituted Furans. Angew. Chem. Int. Ed. 2007, 46, 5195–5197; (c) Dudnik, A. S.; Sromek, A. W.; Rubina, M.; Kim, J. T.; Kel'in, A. V.; Gevorgyan, V. Metal-Catalyzed 1,2-Shift of Diverse Migrating Groups in Allenyl Systems as a New Paradigm toward Densely Functionalized Heterocycles. J. Am. Chem. Soc. 2008, 130, 1440–1452; (d) Dudnik, A. S.; Xia, Y.; Li, Y.; Gevorgyan, V. Computation-Guided Development of Au-Catalyzed Cycloisomerizations Proceeding via 1,2-Si or 1,2-H Migrations: Regiodivergent Synthesis of Silylfurans. J. Am. Chem. Soc. 2010, 132, 7645–7655.
- 7 Miao, M.; Cao, J.; Zhang, J.; Huang, X.; Wu, L. PdCl2-Catalyzed Oxidative Cycloisomerization of 3-Cyclopropylideneprop-2-en-1-ones. Org. Lett. 2012, 14, 2718–2721.
- 8(a) Ma, S.; Zhang, J. Pd0-Catalyzed Cyclization Reaction of Aryl or Alk-1-enyl Halides with 1,2-Dienyl Ketones: A General and Efficient Synthesis of Polysubstituted Furans. Chem. Commun. 2000, 117–118;
(b) Ma, S.; Li, L. Palladium-Catalyzed Cyclization Reaction of Allylic Bromides with 1,2-Dienyl Ketones. An Efficient Synthesis of 3-Allylic Polysubstituted Furans. Org. Lett. 2000, 2, 941–944;
(c) Ma, S.; Yu, Z. Oxidative Cyclization-Dimerization Reaction of 2,3-Allenoic Acids and 1,2-Allenyl Ketones: An Efficient Synthesis of 4-(3’-Furanyl)butenolide Derivatives. Angew. Chem. Int. Ed. 2002, 41, 1775–1778;
10.1002/1521-3773(20020517)41:10<1775::AID-ANIE1775>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar(d) Ma, S.; Zhang, J.; Lu, L. Pd0-Catalyzed Coupling Cyclization Reaction of Aryl or 1-Alkenyl Halides with 1,2-Allenyl Ketones: Scope and Mechanism. An Efficient Assembly of 2,3,4-, 2,3,5-Tri- and 2,3,4,5-Tetrasubstituted Furans. Chem. - Eur. J. 2003, 9, 2447–2456.
- 9For reviews of catalytic carbene coupling reactions, see: (a) Zhang, Y.; Wang, J. Recent Developments in Pd-Catalyzed Reactions of Diazo Compounds. Eur. J. Org. Chem. 2011, 1015–1026; (b) Barluenga, J.; Valdés, C. Tosylhydrazones: New Uses for Classic Reagents in Palladium-Catalyzed Cross-Coupling and Metal-Free Reactions. Angew. Chem. Int. Ed. 2011, 50, 7486–7500; (c) Shao, Z.; Zhang, H. N-Tosylhydrazones: Versatile Reagents for Metal-Catalyzed and Metal-Free Cross-Coupling Reactions. Chem. Soc. Rev. 2012, 41, 560–572; (d) Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889; (e) Xia, Y.; Wang, J. Transition-Metal-Catalyzed Cross-Coupling with Ketones or Aldehydes via N-Tosylhydrazones. J. Am. Chem. Soc. 2020, 142, 10592–10605; (f) Xiao, Q.; Zhang, Y.; Wang, J. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc. Chem. Res. 2013, 46, 236–247; (g) Wang, K.; Wang, J. Transition-Metal-Catalyzed Cross- Coupling with Non-Diazo Carbene Precursors. Synlett 2019, 30, 542–551.
- 10 Xia, Y.; Xia, Y.; Ge, R.; Liu, Z.; Xiao, Q.; Zhang, Y.; Wang, J. Oxidative Cross-Coupling of Allenyl Ketones and Organoboronic Acids: Expeditious Synthesis of Highly Substituted Furans. Angew. Chem. Int. Ed. 2014, 53, 3917–3921.
- 11 Wang, K.; Xu, Y.; Wang, J. Palladium-Catalyzed Cyclizative Borylation of Allenyl Ketones through Carbene Boryl Migratory Insertion: Access to Densely Substituted Furyl Boronates. Chem. - Eur. J. 2023, 29, e202203697.
- 12(a) Diederich, F.; Stang, P. J.; Tykwinski, R. R. Acetylene Chemistry: Chemistry,
Biology and Material Science, Wiley-VCH, Weinheim, 2005;
10.1002/3527605487 Google Scholar(b) Trost, B. M.; Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations, Wiley-VCH, Weinheim, 2015.
- 13For selected examples, see: (a) Zhao, G.; Wu, Y.; Wu, H.-H.; Yang, J.; Zhang, J. Pd/GF-Phos-Catalyzed Asymmetric Three-Component Coupling Reaction to Access Chiral Diarylmethyl Alkynes. J. Am. Chem. Soc. 2021, 143, 17983–17988; (b) Zhou, L.; Ye, F.; Zhang, Y.; Wang, J. Pd-Catalyzed Three-Component Coupling of N-Tosylhydrazone, Terminal Alkyne, and Aryl Halide. J. Am. Chem. Soc. 2010, 132, 13590–13591; (c) Xiao, Q.; Xia, Y.; Li, H.; Zhang, Y.; Wang, J. Coupling of N-Tosylhydrazones with Terminal Alkynes Catalyzed by Copper(I): Synthesis of Trisubstituted Allenes. Angew. Chem. Int. Ed. 2011, 50, 1114–1117; (d) Zhou, L.; Ye, F.; Ma, J.; Zhang, Y.; Wang, J. Palladium-Catalyzed Oxidative Cross-Coupling of N-Tosylhydrazones or Diazoesters with Terminal Alkynes: A Route to Conjugated Enynes. Angew. Chem. Int. Ed. 2011, 50, 3510–3514; (e) Ye, F.; Ma, X.; Xiao, Q.; Li, H.; Zhang, Y.; Wang, J. C(sp)−C(sp3) Bond Formation through Cu-Catalyzed Cross-Coupling of N-Tosylhydrazones and Trialkylsilylethynes. J. Am. Chem. Soc. 2012, 134, 5742–5745; (f) Hossain, M. L.; Ye, F.; Liu, Z.; Xia, Y.; Shi, Y.; Zhou, L.; Zhang, Y.; Wang, J. Synthesis of Phenanthrenes through Copper-Catalyzed Cross-Coupling of N-Tosylhydrazones with Terminal Alkynes. J. Org. Chem. 2014, 79, 8689–8699; (g) Chu, W.-D.; Zhang, L.; Zhang, Z.; Zhou, Q; Mo, F.; Zhang, Y.; Wang, J. Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 14558–14561; (h) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Three- Component Coupling of Trifluoromethyl Ketone N-Tosylhydrazones, Alkynes and Azides: Synthesis of Difluoromethylene Substituted 1,2,3-Triazoles. Chin. J. Chem. 2017, 35, 387–391.
- 14(a) Li, Y.; Brand, J. P.; Waser, J. Gold-Catalyzed Regioselective Synthesis of 2- and 3-Alkynyl Furans. Angew. Chem. Int. Ed. 2013, 52, 6743–6747; (b) Ma, Y.; Zhang, S.; Yang, S.; Song, F.; You, J. Gold-Catalyzed C(sp3)–H/C(sp)–H Coupling/Cyclization/Oxidative Alkynylation Sequence: A Powerful Strategy for the Synthesis of 3-Alkynyl Polysubstituted Furans. Angew. Chem. Int. Ed. 2014, 53, 7870–7874; (c) Han, C.; Tian, X.; Song, L.; Liu, Y.; Hashmi, A. S. K. Tetra-Substituted Furans by A Gold-Catalysed Tandem C(sp3)–H Alkynylation/Oxy-Alkynylation Reaction. Org. Chem. Front. 2021, 8, 6546–6552; (d) Hu, L.; Dietl, M. C.; Han, C.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Au-Ag Bimetallic Catalysis: 3-Alkynyl Benzofurans from Phenols via Tandem C–H Alkynylation/Oxy-Alkynylation. Angew. Chem. Int. Ed. 2021, 60, 10637–10642.
- 15 Jie, X.; Shang, Y.; Hu, P.; Su, W. Palladium-Catalyzed Oxidative Cross-Coupling between Heterocycles and Terminal Alkynes with Low Catalyst Loading. Angew. Chem. Int. Ed. 2013, 52, 3630–3633.
- 16(a) Wang, K.; Xu, Y.; Wang, J. Palladium-Catalyzed Cyclizative Borylation of Allenyl Ketones through Carbene Boryl Migratory Insertion: Access to Densely Substituted Furyl Boronates. Chem. - Eur. J. 2023, 29, e202203697; (b) Petasis, N. A.; Teets, K. A. Enolates of α-Allenyl Ketones: Formation and Aldol Reactions of Cumulenolates. J. Am. Chem. Soc. 1992, 114, 10328–10334; (c) Ma, S.; Zhang, J.; Lu, L. Pd0-Catalyzed Coupling Cyclization Reaction of Aryl or 1-Alkenyl Halides with 1,2-Allenyl Ketones: Scope and Mechanism. An Efficient Assembly of 2,3,4-, 2,3,5-Tri- and 2,3,4,5-Tetrasubstituted Furans. Chem. - Eur. J. 2003, 9, 2447–2456; (d) Ma, S.; Yang, Y.-C.; Toy, P. H. Rasta Resin-TBD-Catalyzed γ-Selective Morita–Baylis–Hillman Reactions of α,γ-Disubstituted Allenones. Synlett 2015, 26, 1732–1736; (e) Teske, J.; Plietker, B. Fe-Catalyzed Cycloisomerization of Aryl Allenyl Ketones: Access to 3-Arylidene-indan-1-ones. Org. Lett. 2018, 20, 2257–2260.
- 17(a) Zorba, L.; Kidonakis, M.; Saridakis, I.; Stratakis, M. Cycloisomerization of Conjugated Allenones into Furans under Mild Conditions Catalyzed by Ligandless Au Nanoparticles. Org. Lett. 2019, 21, 5552–5555; (b) Malhotra, D.; Liu, L.-P.; Hammond, G. B. Tandem Michael Addition/Aldol Reaction of Allenic Ketones with Alkyl Vinyl Ketones: Versatile Synthesis of 2-Alkynyl 1,5-Diketones, 4-Alkynyl-3-hydroxycyclohexanones and 4-Alkynylcyclohexenones. Eur. J. Org. Chem. 2010, 6855–6862; (c) Kusakabe, T.; Mochida, T.; Ariyama, T.; Lee, D.; Ohkubo, S.; Takahashi, K.; Kato, K. PdII Catalyzed Ligand Controlled Synthesis of Bis(3-furanyl)methanones and Methyl 3-furancarboxylates. Org. Biomol. Chem. 2019, 17, 6860–6865; (d) Cheng, C.; Sun, X.; Wu, Z.; Liu, Q.; Xiong, L.; Miao, Z. Lewis Base Catalyzed Regioselective Cyclization of Allene Ketones or α-Methyl Allene Ketones with Unsaturated Pyrazolones. Org. Biomol. Chem. 2019, 17, 323–3238; (e) Zhai, S.; Zhang, X.; Cheng, B.; Li, H.; Li, Y.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of Tetrasubstituted Thiophenes via a [3+2] Cascade Cyclization Reaction of Pyridinium 1,4-Zwitterionic Thiolates and Activated Allene. Chem. Commun. 2020, 56, 3085–3088.




