Facile Synthesis of S-Fused Multi-Membered Polycyclic Heterocycles: A Construction Strategy towards Thermally Stable Thiepine Derivatives
Binbin Ming
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorChuan Yan
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorShoudong Xie
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorSi Liu
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorYingjian Ren
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorHao Zong
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorWeinan Chen
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Gang Zhou
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this authorBinbin Ming
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorChuan Yan
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorShoudong Xie
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorSi Liu
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorYingjian Ren
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorHao Zong
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorWeinan Chen
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Gang Zhou
Lab of Advanced Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
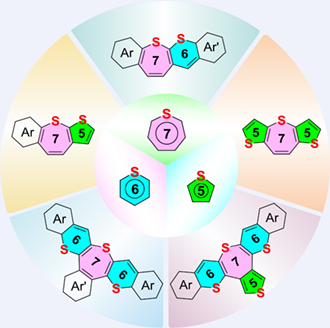
S-fused heterocycles have become popular building blocks to construct functional polycyclic compounds. In contrast to the abundant synthetic methodologies for thiophene-fused aromatics, the synthesis of S-heterocycles containing six-membered thiopyran and seven-membered thiepine rings is much less reported owing to their unfavorable synthetic protocols and the thermal instabilities. Herein, a series of thiepine-containing polycyclic S-heterocycles have been successfully synthesized via different synthetic routes which involve initial construction of sulfur bridges and final ring-closure reactions. Therefore, the dilithium intermediates are excluded, which facilitates the fusion on the thiepine ring with different S-heterocycles, including thiophene and thiopyran derivatives. Typically, a S-fused multi-membered polycyclic compound simultaneously involving thiophen, thiopyran, and thiepine rings has been successfully prepared. Interestingly, nucleus-independent chemical shift calculations reveal that the incorporated thiopyran and thiepine rings demonstrate aromatic and nonaromatic characteristics, respectively. Moreover, the thermal stabilities of the thiepine derivatives have been tremendously improved after the fusion on the three vinyl groups in the thiepine unit, which is attributed to the enhancements of the activation energies for the S-extrusion reactions, as revealed by density functional theory calculations. Therefore, our findings not only provide a facile synthetic methodology for S-fused multi-membered polycyclic heterocycles, but also furnish a novel construction strategy towards thermally stable thiepine derivatives.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200518-sup-0001-Supinfo.pdfPDF document, 4.6 MB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Borissov, A.; Maurya, Y. K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788.
- 2 Li, B.; Peng, W.; Luo, S.; Jiang, C.; Guo, J.; Xie, S.; Hu, Y.; Zhang, Y.; Zeng, Z. Diagonally π-Extended Perylene-Based Bis(heteroacene) for Chiroptical Activity and Integrating Luminescence with Carrier- Transporting Capability. Org. Lett. 2019, 21, 1417–1421.
- 3 Beaujuge, P. M.; Frechet, J. M. J. Molecular Design and Ordering Effects in π-Functional Materials for Transistor and Solar Cell Applications. J. Am. Chem. Soc. 2011, 133, 20009–20029.
- 4 Yang, Y.; Liu, Z.; Zhang, G.; Zhang, X.; Zhang, D. The Effects of Side Chains on the Charge Mobilities and Functionalities of Semiconducting Conjugated Polymers beyond Solubilities. Adv. Mater. 2019, 31, 1903104–1903135.
- 5 Feng, Z.; Cheng, Z.; Jin, H.; Lu, P. Recent Progress of Sulphur-containing High-efficiency Organic Light-Emitting Diodes (OLEDs). J. Mater. Chem. C 2022, 10, 4497–4520.
- 6 Xu, G.; Rao, H.; Liao, X.; Zhang, Y.; Wang, Y.; Xing, Z.; Hu, T.; Tan, L.; Chen, L.; Chen, Y. Reducing Energy Loss and Morphology Optimization Manipulated by Molecular Geometry Engineering for Hetero- junction Organic Solar Cells. Chin. J. Chem. 2020, 38, 1553–1559.
- 7 Yang, L.; Yin, C.-Z.; Ali, M. A.; Dong, C.-Y.; Xie, X.-M.; Wu, X.-P.; Mao, J.; Wang, Y.-X.; Yu, Y.; Xie, L.-H.; Bian, L.-Y. Bao, J.-M.; Ran, X.-Q.; Huang, W. Theoretical Studies on Novel Gridspiroarenes: Structures, Noncovalent Interactions and Reorganization Energies. Chin. J. Chem. 2019, 37, 915–921.
- 8 Lu, Y.; Qiao, Y.; Xue, H.; Zhou, G. From Colorless to Near-Infrared S-Heteroarene Isomers: Unexpected Cycloaromatization of Cyclopenta[b]thiopyran Catalyzed by PtCl2. Org. Lett. 2018, 20, 6632–6635.
- 9 Song, C.; Swager, T. M. Reactive Conducting Thiepin Polymers. J. Org. Chem. 2010, 75, 999–1005.
- 10 Liu, C.; Ni, Y.; Lu, X.; Li, G.; Wu, J. Global Aromaticity in Macrocyclic Polyradicaloids: Huckel's Rule or Baird's Rule? Acc. Chem. Res. 2019, 52, 2309–2321.
- 11 Saranya, P. V.; Neetha, M.; Radhika, S.; Anilkumar, G. An Overview of Palladium-Catalyzed Synthesis of Seven-Membered Heterocycles. J. Heterocycl. Chem. 2020, 58, 673–684.
- 12 Bozinovic, N.; Segan, S.; Vojnovic, S.; Pavic, A.; Solaja, B. A.; Nikodinovic-Runic, J.; Opsenica, I. M. Synthesis and Anti-Candida Activity of Novel Benzothiepino[3,2-c]pyridine Derivatives. Chem. Biol. Drug Des. 2016, 88, 795–806.
- 13 Shen, S.; Zhang, H.; Yang, W.; Yu, C.; Yao, C. One-pot Combinatorial Synthesis of Benzo[4,5]imidazo-[1,2-a]thiopyrano [3,4-d]pyrimidin- 4(3H)-one Derivatives. Chin. J. Chem. 2011, 29, 1727–1731.
- 14 Wang, D.; Xiao, F.; Zhang, F.; Deng G-J. Three-Component Synthesis of 2-Heteroaryl-3-hydroxybenzo[b]thiophenes under Transition- Metal-Free Conditions. Chin. J. Chem. 2021, 39, 2483–2488.
- 15 Tahara, Y.-K.; Matsubara, R.; Mitake, A.; Sato, T.; Kanyiva, K. S.; Shibata, T. Catalytic and Enantioselective Synthesis of Chiral Multisubstituted Tribenzothiepins by Intermolecular Cycloadditions. Angew. Chem. Int. Ed. 2016, 55, 4552–4556.
- 16 Inami, T.; Takahashi, T.; Kurahashi, T.; Matsubara, S. Nickel-Catalyzed [5+2] Cycloaddition of 10π-Electron Aromatic Benzothiophenes with Alkynes to Form Thermally Metastable 12π-Electron Nonaromatic Benzothiepines. J. Am. Chem. Soc. 2019, 141, 12541–12544.
- 17 Sprenger, K.; Golz, C.; Alcarazo, M. Synthesis of Cycloheptatrienes, Oxepines, Thiepines, and Silepines: A Comparison between Brønsted Acid and Au-Catalysis. Eur. J. Org. Chem. 2020, 2020, 6245–6254.
- 18 Hayakawa, S.; Matsuo, K.; Yamada, H.; Fukui, N.; Shinokubo, H. Dinaphthothiepine Bisimide and Its Sulfoxide: Soluble Precursors for Perylene Bisimide. J. Am. Chem. Soc. 2020, 142, 11663–11668.
- 19 Rajca, A.; Miyasaka, M.; Pink, M.; Wang, H.; Rajca, S. Helically Annelated and Cross-Conjugated Oligothiophenes: Asymmetric Synthesis, Resolution, and Characterization of a Carbon-Sulfur [7]Helicene. J. Am. Chem. Soc. 2004, 126, 15211–15222.
- 20 Jiang, M.; Li, H.; Yang, H.; Fu, H. Room-Temperature Arylation of Thiols: Breakthrough with Aryl Chlorides. Angew. Chem. Int. Ed. 2017, 56, 874–879.
- 21 Ansari, M. I.; Hussain, M. K.; Arun, A.; Chakravarti, B.; Konwar, R.; Hajela, K. Synthesis of Targeted Dibenzo[b,f]thiepines and Dibenzo[b,f]oxepines as Potential Lead Molecules with Promising Anti-breast Cancer Activity. Eur. J. Med. Chem. 2015, 99, 113–124.
- 22 Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483.
- 23 Qiao, Y.; Lu, Y.; Chen, W.; Chen, Y.; Baumgarten, M.; Zhou, G. Cyclopenta[b]thiopyran and Cyclopenta[b]selenopyran Based Heteroarenes: Electronic Communication between S- and/or Se-Fused Aromatics. Chem. Commun. 2019, 55, 5107–5110.
- 24 Chadwick, D. J.; Chambers, J.; Hargraves, H. E.; Meakins, G. D.; Snowden, R. L. Preparation of Substituted Furan- and Thiophen-2- carbaldehydes and -2-[2H]carbaldehydes, and of 2-Furyl Ketones. J. Chem. Soc., Perkin Trans. 1 1973, 2327–2332.
- 25 Oechsle, P.; Paradies, J. Ambidextrous Catalytic Access to Dithieno[3,2-b:2’,3’-d]thiophene (DTT) Derivatives by Both Palladium-Catalyzed C-S and Oxidative Dehydro C-H Coupling. Org. Lett. 2014, 16, 4086–4089.
- 26 Shirani, H.; Janosik, T. A New Concise Strategy for Synthesis of Dibenzo[b,f]thiepins and Related Fused Symmetrical Thiepin Derivatives. J. Org. Chem. 2007, 72, 8984–8986.
- 27 Kinzel, T.; Zhang, Y.; Buchwald, S. L. A New Palladium Precatalyst Allows for the Fast Suzuki-Miyaura Coupling Reactions of Unstable Polyfluorophenyl and 2-Heteroaryl Boronic Acids. J. Am. Chem. Soc. 2010, 132, 14073–14075.
- 28 Hoffman, J. M. Jr.; Schlessinger, R. H. Synthesis of a Stable 8-π-Electron Thiepin. J. Am. Chem. Soc. 1970, 92, 5263–5265.
- 29 Kitamura, T.; Soda, S.-i.; Kawasato, H.; Taniguchi, H.; Shiro, M. Intramolecular Cyclization of [o-(Arylthio)phenyl]ethenes. Synthesis and Crystal Structure of 1-Arylbenzo[b]thiophenium Salts. Tetrahedron 1993, 49, 5055–5066.
- 30 Seppelt, K. Fluorine-Stabilized Sulfur-Carbon Multiple Bonds. Angew. Chem. Int. Ed. Engl. 1991, 30, 361–374.
- 31 Liu, R. S. H. Colorful Azulene and Its Equally Colorful Derivatives. J. Chem. Educ. 2002, 79, 183–185.
- 32 Englman, R.; Jortner, J. The Energy Gap Law for Radiationless Transitions in Large Molecules. Mol. Phys. 1970, 18, 145–164.
- 33Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A.; Peralta, Jr. J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2019.
- 34 Stanger, A. Nucleus-Independent Chemical Shifts (NICS): Distance Dependence and Revised Criteria for Aromaticity and Antiaromaticity. J. Org. Chem. 2006, 71, 883–893.
- 35 Mardirossian, N.; Head-Gordon, M. ωB97M-V: A Combinatorially Optimized, Range-separated Hybrid, Meta-GGA Density Functional with VV10 Nonlocal Correlation. J. Chem. Phys. 2016, 144, 214110–214132.
- 36 Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.




