Tuning the Reactivity of Cyclopropenes from Living Ring-Opening Metathesis Polymerization (ROMP) to Single-Addition and Alternating ROMP
Jessica K. Su
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorZexin Jin
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorRui Zhang
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorGang Lu
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng Liu
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Yan Xia
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorJessica K. Su
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorZexin Jin
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorRui Zhang
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorGang Lu
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng Liu
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, 15260 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Yan Xia
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorGraphical Abstract
Abstract
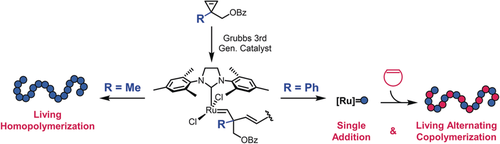
Ring-opening metathesis polymerization (ROMP) has become one of the most important living polymerizations. Cyclopropenes (CPEs) remain underexplored for ROMP. Described here is that the simple swap of 1-methyl to 1-phenyl on 1-(benzoyloxymethyl)CPEs elicited strikingly different modes of reactivity, switching from living polymerization to either selective single-addition or living alternating ROMP. The distinct reactivity stems from differences in steric repulsions at the Ru alkylidene after CPE ring opening. Possible olefin or oxygen chelation from ring-opened CPE substituents was also observed to significantly affect the rate of propagation. These results demonstrate the versatility of CPEs as a new class of monomers for ROMP, provide mechanistic insights for designing new monomers with rare single-addition reactivity, and generate a new functionalizable alternating copolymer scaffold with controlled molecular weight and low dispersity.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909688-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Leitgeb, J. Wappel, C. Slugovc, Polymer 2010, 51, 2927–2946;
- 1bR. R. Schrock, Acc. Chem. Res. 2014, 47, 2457–2466;
- 1c Handbook of Metathesis, Vol. 3 , 2nd ed., Wiley-VCH, Weinheim, 2015.
- 2
- 2aB. R. Maughon, R. H. Grubbs, Macromolecules 1997, 30, 3459–3469;
- 2bK. A. Parker, N. S. Sampson, Acc. Chem. Res. 2016, 49, 408–417;
- 2cH. Martinez, N. Ren, M. E. Matta, M. A. Hillmyer, Polym. Chem. 2014, 5, 3507–3532;
- 2dM. A. Hillmyer, S. T. Nguyen, R. H. Grubbs, Macromolecules 1997, 30, 718–721;
- 2eK. Kratz, K. Breitenkamp, R. Hule, D. Pochan, T. Emrick, Macromolecules 2009, 42, 3227–3229;
- 2fR. Walker, R. M. Conrad, R. H. Grubbs, Macromolecules 2009, 42, 599–605;
- 2gS. Kobayashi, L. M. Pitet, M. A. Hillmyer, J. Am. Chem. Soc. 2011, 133, 5794–5797;
- 2hO. A. Scherman, R. H. Grubbs, Synth. Met. 2001, 124, 431–434;
- 2iZ. Chen, J. A. M. Mercer, X. Zhu, J. A. H. Romaniuk, R. Pfattner, L. Cegelski, T. J. Martinez, N. Z. Burns, Y. Xia, Science 2017, 357, 475–479;
- 2jJ. Yang, M. Horst, J. A. H. Romaniuk, Z. Jin, L. Cegelski, Y. Xia, J. Am. Chem. Soc. 2019, 141, 6479–6483.
- 3J. F. Liebman, A. Greenberg, Chem. Rev. 1976, 76, 311–365.
- 4
- 4aR. Singh, C. Czekelius, R. R. Schrock, Macromolecules 2006, 39, 1316–1317;
- 4bR. Singh, R. R. Schrock, Macromolecules 2008, 41, 2990–2993;
- 4cW. H. Binder, S. Kurzhals, B. Pulamagatta, U. Decker, G. Manohar Pawar, D. Wang, C. Kühnel, M. R. Buchmeiser, Macromolecules 2008, 41, 8405–8412;
- 4dM. M. Flook, L. C. H. Gerber, G. T. Debelouchina, R. R. Schrock, Macromolecules 2010, 43, 7515–7522.
- 5
- 5aB. R. Elling, Y. Xia, J. Am. Chem. Soc. 2015, 137, 9922–9926;
- 5bB. R. Elling, J. K. Su, J. D. Feist, Y. Xia, Chem 2019, 5, 2691–2701.
- 6
- 6aA. Song, K. A. Parker, N. S. Sampson, J. Am. Chem. Soc. 2009, 131, 3444–3445;
- 6bL. Tan, K. A. Parker, N. S. Sampson, Macromolecules 2014, 47, 6572–6579;
- 6cL. Tan, G. Li, K. A. Parker, N. S. Sampson, Macromolecules 2015, 48, 4793–4800;
- 6dD. Oh, M. Ouchi, T. Nakanishi, H. Ono, M. Sawamoto, ACS Macro Lett. 2016, 5, 745–749;
- 6eJ. Xu, C. Fu, S. Shanmugam, C. J. Hawker, G. Moad, C. Boyer, Angew. Chem. Int. Ed. 2017, 56, 8376–8383; Angew. Chem. 2017, 129, 8496–8503;
- 6fZ. Huang, B. B. Noble, N. Corrigan, Y. Chu, K. Satoh, D. S. Thomas, C. J. Hawker, G. Moad, M. Kamigaito, M. L. Coote, C. Boyer, J. Xu, J. Am. Chem. Soc. 2018, 140, 13392–13406.
- 7
- 7a“Sequence-Controlled Polymers by Ruthenium-Mediated Ring-Opening Metathesis Polymerization”: A. B. Chang, G. M. Miyake, R. H. Grubbs in Sequence-Controlled Polymers: Synthesis Self-Assembly, and Properties, Vol. 1170, American Chemical Society, Washington, 2014, pp. 161–188;
- 7bM. F. Ilker, E. B. Coughlin, Macromolecules 2002, 35, 54–58;
- 7cT.-L. Choi, I. M. Rutenberg, R. H. Grubbs, Angew. Chem. Int. Ed. 2002, 41, 3839–3841;
10.1002/1521-3773(20021018)41:20<3839::AID-ANIE3839>3.0.CO;2-H CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3995–3997;
- 7dK. Vehlow, D. Wang, M. R. Buchmeiser, S. Blechert, Angew. Chem. Int. Ed. 2008, 47, 2615–2618; Angew. Chem. 2008, 120, 2655–2658;
- 7eS. Torker, A. Müller, R. Sigrist, P. Chen, Organometallics 2010, 29, 2735–2751;
- 7fR. Vasiuta, A. Stockert, H. Plenio, Chem. Commun. 2018, 54, 1706–1709;
- 7gM. M. Flook, V. W. L. Ng, R. R. Schrock, J. Am. Chem. Soc. 2011, 133, 1784–1786;
- 7hH. Jeong, J. M. John, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2015, 137, 2239–2242;
- 7iE. S. Jang, J. M. John, R. R. Schrock, ACS Cent. Sci. 2016, 2, 631–636;
- 7jE. S. Jang, J. M. John, R. R. Schrock, J. Am. Chem. Soc. 2017, 139, 5043–5046.
- 8G. Li, N. S. Sampson, Macromolecules 2018, 51, 3932–3940.
- 9
- 9aD. N. Kamber, L. A. Nazarova, Y. Liang, S. A. Lopez, D. M. Patterson, H.-W. Shih, K. N. Houk, J. A. Prescher, J. Am. Chem. Soc. 2013, 135, 13680–13683;
- 9bR. Selvaraj, S. R. Chintala, M. T. Taylor, J. M. Fox, Org. Synth. 2014, 91, 322–337.
- 10B. R. Elling, Y. Xia, ACS Macro Lett. 2018, 7, 656–661.
- 11
- 11aE. A. Ofstead, N. Calderon, Makromol. Chem. 1972, 154, 21–34;
- 11bL. Reif, H. Hoecker, Macromolecules 1984, 17, 952–956;
- 11cZ.-R. Chen, J. P. Claverie, R. H. Grubbs, J. A. Kornfield, Macromolecules 1995, 28, 2147–2154.
- 12J.-A. Song, B. Park, S. Kim, C. Kang, D. Lee, M.-H. Baik, R. H. Grubbs, T.-L. Choi, J. Am. Chem. Soc. 2019, 141, 10039–10047.
- 13S. B. Garber, J. S. Kingsbury, B. L. Gray, A. H. Hoveyda, J. Am. Chem. Soc. 2000, 122, 8168–8179.
- 14The [2+2] cycloaddition to form ruthenacyclobutane is the rate-determining step in ROMP of strained cycloalkenes, such as cyclobutenes, cyclopentenes, and norbornenes. See:
- 14aP. Chen, C. Adlhart, J. Am. Chem. Soc. 2004, 126, 3496–3510;
- 14bH. Martinez, P. Miró, P. Charbonneau, M. A. Hillmyer, C. J. Cramer, ACS Catal. 2012, 2, 2547–2556;
- 14cC. N. Rowley, E. F. van der Eide, W. E. Piers, T. K. Woo, Organometallics 2008, 27, 6043–6045;
- 14dD. Benitez, E. Tkatchouk, W. A. Goddard III, Organometallics 2009, 28, 2643–2645.
- 15
- 15aD. H. Ess, K. N. Houk, J. Am. Chem. Soc. 2008, 130, 10187–10198;
- 15bB. F. Matthias, K. N. Houk, Angew. Chem. Int. Ed. 2017, 56, 10070–10086; Angew. Chem. 2017, 129, 10204–10221.
- 16The n-pentyl group in 4 was replaced with a methyl in the calculations for simplicity.
- 17DFT calculations suggested the bottom-bound E-selective transition states are the lowest in energy in reactions of alkylidenes 6 and 8. See the Supporting Information for details. This result is consistent with previous computational studies that suggested a bottom-bound pathway:
- 17aS. E. Vyboishchikov, M. Buhl, W. Thiel, Chem. Eur. J. 2002, 8, 3962–3975;
10.1002/1521-3765(20020902)8:17<3962::AID-CHEM3962>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 17bL. Cavallo, A. Correa, J. Am. Chem. Soc. 2006, 128, 13352–13353;
- 17cD. Benitez, E. Tkatchouk, W. A. Goddard, Chem. Commun. 2008, 6194–6196.
- 18The isomers of 6 b and 8 b in which the ester carbonyl oxygen atom binds to the Ru are less stable, because these isomers involve a less favorable seven-membered chelate rather than the five-membered chelate in 6 b and 8 b (see Figure S40 for details). For a computational study on the effects of ester coordination in ROMP of cyclobutene derivatives, see: A. Song, J. C. Lee, K. A. Parker, N. S. Sampson, J. Am. Chem. Soc. 2010, 132, 10513–10520.
- 19CCDC 1583892 (Ru-5) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre