The Cyano Group as a Traceless Activation Group for the Intermolecular [3+2] Cycloaddition of Azomethine Ylides: A Five-Step Synthesis of (±)-Isoretronecanol†
Jundong Li
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorHuaibo Zhao
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorXunjin Jiang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorXiance Wang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorHaiming Hu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorLei Yu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yandong Zhang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)Search for more papers by this authorJundong Li
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorHuaibo Zhao
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorXunjin Jiang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorXiance Wang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorHaiming Hu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorLei Yu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yandong Zhang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005 (P.R. China)Search for more papers by this authorThis research was supported by the Opening Foundation of the Laboratory of Chemical Genomics of Peking University Shenzhen Graduate School, the NSFC (21142007 and 21272194), the RFDP (20110121120014), the NSFFJ (2012J01055), the NFFTBS (J1310024), the FRFCU (2012121017), the SRF for ROCS of SEM, and the Program for Changjiang Scholars and Innovative Research Teams in University (PCSIRT). We also thank Prof. Tingbin Wen and Jie Ouyang for the help with X-ray crystallographic analysis and 11B NMR spectroscopic experiments.
Graphical Abstract
Abstract
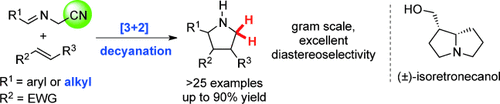
The cyano group was used as a traceless activation group for the [3+2] cycloaddition of azomethine ylides in a two-step process, thereby providing a highly effective approach to 5-unsubstituted pyrrolidines. The transformation includes the silver acetate catalyzed intermolecular 1,3-dipolar cycloaddition of α-iminonitriles and an unprecedented sodium borohydride induced reductive decyanation reaction. A diverse array of substrates is amenable to this transformation. The methodology was further extended to a five-step total synthesis of the pyrrolizidine natural product isoretronecanol.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201500961_sm_miscellaneous_information.pdf5.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Buckingham, K. H. Baggaley, A. D. Roberts, L. F. Szabó, Dictionary of Alkaloids, 2nd ed. with CD-ROM, CRC, Boca Raton, FL, 2010;
10.1201/EBK1420077698 Google Scholar
- 1bE. Fattorusso, O. Taglialatela-Scafati, Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH, Weinheim, 2008.
- 2For reviews, see:
- 2aC. M. R. Volla, I. Atodiresei, M. Rueping, Chem. Rev. 2014, 114, 2390–2431;
- 2bP. Pihko, I. Majander, A. Erkkilä in Asymmetric Organocatalysis, Vol. 291 (Ed.: ), Springer, Berlin, 2009, pp. 145–200;
- 2cS. Bertelsen, K. A. Jørgensen, Chem. Soc. Rev. 2009, 38, 2178–2189.
- 3
- 3aE. Poupon, R. Salame, L.-H. Yan, Biomimetic Organic Synthesis, Wiley-VCH, Weinheim, 2011, pp. 1–60;
10.1002/9783527634606.ch1 Google Scholar
- 3bP. M. Dewick, Medicinal Natural Products, Wiley, Hoboken, 2009, pp. 311–420.
10.1002/9780470742761.ch6 Google Scholar
- 4
- 4aA. Pascual-Escudero, M. González-Esguevillas, S. Padilla, J. Adrio, J. C. Carretero, Org. Lett. 2014, 16, 2228–2231;
- 4bJ. Hernández-Toribio, S. Padilla, J. Adrio, J. C. Carretero, Angew. Chem. Int. Ed. 2012, 51, 8854–8858; Angew. Chem. 2012, 124, 8984–8988.
- 5For reviews, see:
- 5aR. Narayan, M. Potowski, Z. J. Jia, A. P. Antonchick, H. Waldmann, Acc. Chem. Res. 2014, 47, 1296–1310;
- 5bJ. Adrio, J. C. Carretero, Chem. Commun. 2014, 50, 12434–12446;
- 5cG. Pandey, P. Banerjee, S. R. Gadre, Chem. Rev. 2006, 106, 4484–4517;
- 5dC. Najera, J. M. Sansano, Curr. Org. Chem. 2003, 7, 1105–1150.
- 6
- 6aW. H. Pearson, B. W. Lian, Angew. Chem. Int. Ed. 1998, 37, 1724–1726;
10.1002/(SICI)1521-3773(19980703)37:12<1724::AID-ANIE1724>3.0.CO;2-8 CAS Web of Science® Google ScholarAngew. Chem. 1998, 110, 1782–1784;10.1002/(SICI)1521-3757(19980619)110:12<1782::AID-ANGE1782>3.0.CO;2-B Web of Science® Google Scholar
- 6bW. H. Pearson, F. E. Lovering, J. Am. Chem. Soc. 1995, 117, 12336–12337;
- 6cW. H. Pearson, D. P. Szura, M. J. Postich, J. Am. Chem. Soc. 1992, 114, 1329–1345.
- 7
- 7aW. H. Pearson, R. B. Clark, Tetrahedron Lett. 1999, 40, 4467–4471;
- 7bW. H. Pearson, Y. Mi, Tetrahedron Lett. 1997, 38, 5441–5444;
- 7cW. H. Pearson, D. P. Szura, W. G. Harter, Tetrahedron Lett. 1988, 29, 761–764;
- 7dK. Achiwa, M. Sekiya, Tetrahedron Lett. 1982, 23, 2589–2592.
- 8For selected examples, see:
- 8aM. Movassaghi, M. A. Schmidt, Angew. Chem. Int. Ed. 2007, 46, 3725–3728; Angew. Chem. 2007, 119, 3799–3802;
- 8bC. Li, C. Chan, A. C. Heimann, S. J. Danishefsky, Angew. Chem. Int. Ed. 2007, 46, 1444–1447; Angew. Chem. 2007, 119, 1466–1469;
- 8cD. Crich, A. B. Pavlovic, R. Samy, Tetrahedron 1995, 51, 6379–6384.
- 9For recent developments, see:
- 9aL. Chu, C. Ohta, Z. Zuo, D. W. C. MacMillan, J. Am. Chem. Soc. 2014, 136, 10886–10889;
- 9bJ. Luo, S. Preciado, I. Larrosa, J. Am. Chem. Soc. 2014, 136, 4109–4112;
- 9cJ. M. Neely, T. Rovis, J. Am. Chem. Soc. 2014, 136, 2735–2738.
- 10
- 10aL. Hu, O. Ramström, Chem. Commun. 2014, 50, 3792–3794;
- 10bR. Robles-Machín, I. Alonso, J. Adrio, J. C. Carretero, Chem. Eur. J. 2010, 16, 5286–5291;
- 10cK. Ueno, S. Kanemasa, O. Tsuge, Bull. Chem. Soc. Jpn. 1989, 62, 808–815;
- 10dO. Tsuge, S. Kanemasa, K. Yorozu, K. Ueno, Bull. Chem. Soc. Jpn. 1987, 60, 3359–3366;
- 10eO. Tsuge, K. Ueno, S. Kanemasa, K. Yorozu, Bull. Chem. Soc. Jpn. 1986, 59, 1809–1824;
- 10fO. Tsuge, S. Kanemasa, K. Yorozu, K. Ueno, Chem. Lett. 1985, 1601–1604.
- 11For a review, see:
- 11aJ. M. Mattalia, C. Marchi-Delapierre, H. Hazimeh, M. Chanon, Arkivoc 2006, 90–118; for selected examples, see:
- 11bC. Kison, T. Opatz, Eur. J. Org. Chem. 2008, 2740–2745;
- 11cS. Shahane, F. Louafi, J. Moreau, J.-P. Hurvois, J.-L. Renaud, P. van de Weghe, T. Roisnel, Eur. J. Org. Chem. 2008, 4622–4631;
- 11dM. B. Sassaman, Tetrahedron 1996, 52, 10835–10840;
- 11eK. Ogura, Y. Shimamura, M. Fujita, J. Org. Chem. 1991, 56, 2920–2922.
- 12For pioneering studies, see:
- 12aH. C. Brown, N. M. Yoon, J. Am. Chem. Soc. 1968, 90, 2686–2688;
- 12bM. W. Rathke, H. C. Brown, J. Am. Chem. Soc. 1966, 88, 2606–2607.
- 13M. Couturier, J. L. Tucker, B. M. Andresen, P. Dubé, J. T. Negri, Org. Lett. 2001, 3, 465–467.
- 14CCDC 1045918 (3 n) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15For anionic chain reactions, see:
- 15aT. Shono, N. Kise, M. Masuda, T. Suzumoto, J. Org. Chem. 1985, 50, 2527–2533;
- 15bT. Shono, H. Ohmizu, S. Kawakami, S. Nakano, N. Kise, Tetrahedron Lett. 1981, 22, 871–874; for anionic chain reactions in polymerization, see:
- 15cG. Odian, Principles of Polymerization, Wiley, Hoboken, 2004, pp. 372–463.
10.1002/047147875X.ch5 Google Scholar
- 16For reviews on amine–borane complexes, see:
- 16aA. Staubitz, A. P. M. Robertson, M. E. Sloan, I. Manners, Chem. Rev. 2010, 110, 4023–4078;
- 16bB. Carboni, L. Monnier, Tetrahedron 1999, 55, 1197–1248.
- 17For reports about imine–borane complexes, see:
- 17aR. J. Burford, M. J. Geier, C. M. Vogels, A. Decken, S. A. Westcott, J. Organomet. Chem. 2013, 731, 1–9;
- 17bJ. A. Pigza, J.-S. Han, A. Chandra, D. Mutnick, M. Pink, J. N. Johnston, J. Org. Chem. 2013, 78, 822–843.
- 18S. Ikegami, S. Yamada, Chem. Pharm. Bull. 1966, 14, 1389–1399.
- 19For previous total syntheses of isoretronecanol, see:
- 19aM. Brambilla, S. G. Davies, A. M. Fletcher, P. M. Roberts, J. E. Thomson, Tetrahedron 2014, 70, 204–211;
- 19bK. Ishii, T. Sone, Y. Shimada, T. Shigeyama, M. Noji, S. Sugiyama, Tetrahedron 2004, 60, 10887–10898.