Carbonylation of Propargyl Carbamates with Palladium(II) Bisoxazoline Catalysts: Efficient Synthesis of 5-Methoxy-3(2H)-furanones†
Dr. Taichi Kusakabe
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorTakeo Takahashi
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorRong Shen
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorAyumi Ikeda
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorYogesh Daulat Dhage
The Institute of Scientific and Industrial Research (ISIR), Osaka University, Mihogaoka, Ibaraki-shi, Osaka 567-0047 (Japan)
Search for more papers by this authorDr. Yuichro Kanno
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorProf. Dr. Yoshio Inouye
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorProf. Dr. Hiroaki Sasai
The Institute of Scientific and Industrial Research (ISIR), Osaka University, Mihogaoka, Ibaraki-shi, Osaka 567-0047 (Japan)
Search for more papers by this authorProf. Dr. Tomoyuki Mochida
Department of Chemistry, Faculty of Sciences, Kobe University, Rokkodai, Nada, Kobe 657-8501 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Keisuke Kato
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)Search for more papers by this authorDr. Taichi Kusakabe
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorTakeo Takahashi
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorRong Shen
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorAyumi Ikeda
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorYogesh Daulat Dhage
The Institute of Scientific and Industrial Research (ISIR), Osaka University, Mihogaoka, Ibaraki-shi, Osaka 567-0047 (Japan)
Search for more papers by this authorDr. Yuichro Kanno
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorProf. Dr. Yoshio Inouye
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Search for more papers by this authorProf. Dr. Hiroaki Sasai
The Institute of Scientific and Industrial Research (ISIR), Osaka University, Mihogaoka, Ibaraki-shi, Osaka 567-0047 (Japan)
Search for more papers by this authorProf. Dr. Tomoyuki Mochida
Department of Chemistry, Faculty of Sciences, Kobe University, Rokkodai, Nada, Kobe 657-8501 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Keisuke Kato
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510 (Japan)Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research (C) (No. 24590026).
Graphical Abstract
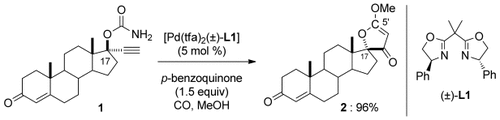
Palladium and CO: Carbonylation of 1 with [Pd(tfa)2(±)-L1] (tfa=trifluoroacetate) affords the spirofuranone 2 with inversion of the stereochemistry at C17 in 96 % yield. C17-epi-1 also gave the same product 2 with retention of the stereochemistry at C17. Labelling studies show that 13CO was incorporated into the C5′ position of the furanone ring. The first asymmetric version of this new reaction was achieved.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303684_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aFor recent review: T. T. Haug, S. F. Kirsch, Targets in Heterocyclic Systemes, Vol. 13 (Ed.: ), Royal Society of Chemistry, London, 2009, p. 57;
- 1bS. M. Kupchan, C. W. Sigel, M. J. Matz, C. J. Gilmore, R. F. Bryan, J. Am. Chem. Soc. 1976, 98, 2295;
- 1cP. K. Chowdhury, R. P. Sharma, G. Thyagarajan, W. Herz, S. V. Govindan, J. Org. Chem. 1980, 45, 4993;
- 1dP. J. Jerris, A. B. Smith III, J. Org. Chem. 1981, 46, 577;
- 1eX. Shao, M. Dolder, C. Tamm, Helv. Chim. Acta 1990, 73, 483;
- 1fG. Comte, D. P. Allais, A. J. Chulia, J. Vercauteren, C. Bosso, Tetrahedron Lett. 1996, 37, 2955;
- 1gJ. He, E. M. K. Wijeratne, B. P. Bashyal, J. Zhan, C. J. Seliga, M. X. Liu, E. E. Pierson, L. S. Pierson III, H. D. VanEtten, A. A. L. Gunatilaka, J. Nat. Prod. 2004, 67, 1985;
- 1hB. Kubze, H. Reichenbach, R. Müller, G. Höfle, J. Antibiot. 2005, 58, 244;
- 1iA. H. Banskota, J. B. McAlpine, D. Sørensen, M. Aouidate, M. Piraee, A. M. Alarco, S. Omura, K. Shiomi, C. M. Farnet, E. Zazopoulos, J. Antibiot. 2006, 59, 168;
- 1jK. C. Nicolaou, D. Sarlah, D. M. Shaw, Angew. Chem. 2007, 119, 4792;
10.1002/ange.200701552 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4708;
- 1kT. Kitamura, T. Takeuchi, Y. Kumamoto-Yonezawa, E. Ohashi, H. Ohmori, C. Masutani, F. Hanaoka, F. Sugawara, H. Yoshida, Y. Mizushina, Bioorg. Med. Chem. Lett. 2009, 17, 1811;
- 1lH. Burghart-Stoll, R. Brückner, Eur. J. Org. Chem. 2012, 3978.
- 2
- 2aA. B. Smith III, P. A. Levenberg, P. J. Jerris, R. M. Scarborough, Jr., P. M. Wovkulich, J. Am. Chem. Soc. 1981, 103, 1501;
- 2bY. Inoue, K. Ohuchi, I. F. Yen, S. Imaizumi, Bull. Chem. Soc. Jpn. 1989, 62, 3518;
- 2cD. Villemin, P. A. Jaffrès, M. Hachémi, Tetrahedron Lett. 1997, 38, 537;
- 2dP. G. Steel, Chem. Commun. 1999, 2257;
- 2eP. Langer, T. Krummel, Chem. Commun. 2000, 967;
- 2fS. Aoki, T. Oi, K. Shimizu, R. Shiraki, K. Takao, K. Tadano, Bull. Chem. Soc. Jpn. 2004, 77, 1703;
- 2gS. F. Kirsch, J. T. Binder, C. Liébert, H. Menz, Angew. Chem. 2006, 118, 6010;
10.1002/ange.200601836 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5878;
- 2hK. Kato, H. Nouchi, K. Ishikura, S. Takaishi, S. Motodate, H. Tanaka, K. Okudaira, T. Mochida, R. Nishigaki, K. Shigenobu, H. Akita, Tetrahedron 2006, 62, 2545;
- 2iY. Liu, M. Liu, S. Guo, H. Tu, Y. Zhou, H. Gao, Org. Lett. 2006, 8, 3445;
- 2jC. M. Marson, E. Edaan, J. M. Morrell, S. J. Coles, M. B. Hursthouse, D. T. Davies, Chem. Commun. 2007, 2494;
- 2kM. Egi, K. Azechi, M. Saneto, K. Shimizu, S. Akai, J. Org. Chem. 2010, 75, 2123;
- 2lC. Gryparis, I. N. Lykakis, C. Efe, I. P. Zaravinos, T. Vidali, E. Kladou, M. Stratakis, Org. Biomol. Chem. 2011, 9, 5655;
- 2mG. Yuan, Z. He, J. Zheng, Z. Chen, H. Huang, D. Shi, C. Qi, H. Jiang, Tetrahedron Lett. 2011, 52, 5956;
- 2nC. Qi, H. Jiang, L. Huang, G. Yuan, Y. Ren, Org. Lett. 2011, 13, 5520;
- 2oS. Amslinger, S. K. Lindner, Synthesis 2011, 2671;
- 2pT. Zarganes-Tzitzikas, M. A. Terzidis, J. Stephanidou-Stephanatou, C. A. Tsoleridis, G. E. Kostakis, J. Org. Chem. 2011, 76, 9008;
- 2qX. Dou, X. Han, Y. Lu, Chem. Eur. J. 2012, 18, 85.
- 3
- 3aR. A. Mack, W. I. Zazulak, L. A. Radov, J. E. Baer, J. D. Stewart, P. H. Elzer, C. R. Kinsolving, V. St. Georgiev, J. Med. Chem. 1988, 31, 1910;
- 3bT. Sasaki, J. Yamakoshi, M. Saito, K. Kasai, T. Matsudo, M. Kikuchi, T. Koga, K. Mori, Biosci. Biotechnol. Biochem. 1998, 62, 2145;
- 3cA. Carotti, A. Carrieri, S. Chimichi, M. Boccalini, B. Cosimelli, C. Gnerre, A. Carotti, P. A. Carrupt, B. Testa, Bioorg. Med. Chem. Lett. 2002, 12, 3551;
- 3dS. Chimichi, M. Boccalini, B. Cosimelli, F. Dall’Acqua, G. Viola, Tetrahedron 2003, 59, 5215;
- 3eS. S. Shin, Y. Byun, K. M. Lim, J. K. Choi, K. W. Lee, J. H. Moh, J. K. Kim, Y. S. Jeong, J. Y. Kim, Y. H. Choi, H. J. Koh, Y. H. Park, Y. I. Oh, M. S. Noh, S. Chung, J. Med. Chem. 2004, 47, 792.
- 4
- 4a Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. II (Ed.: ), Wiley, New York, 2002, p. 2309. For recent reviews, see:
- 4bY. Yamamoto, Chem. Rev. 2012, 112, 4736;
- 4cB. Godoi, R. F. Schumacher, G. Zeni, Chem. Rev. 2011, 111, 2937;
- 4dC. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 2011, 111, 1954;
- 4eR. Grigg, S. P. Mutton, Tetrahedron 2010, 66, 5515;
- 4fK. C. Majumdar, B. Chattopadhyay, P. K. Maji, S. K. Chattopadhyay, S. Samanta, Heterocycles 2010, 81, 795;
- 4gK. C. Majumdar, P. Debnath, B. Roy, Heterocycles 2009, 78, 2661;
- 4hN. Asao, Synlett 2006, 1645;
- 4iS. Ma, Chem. Rev. 2005, 105, 2829;
- 4jJ. Muzart, Tetrahedron 2005, 61, 5955;
- 4kB. C. G. Söderberg, Coord. Chem. Rev. 2004, 248, 1085;
- 4lG. Zeni, R. C. Larock, Chem. Rev. 2004, 104, 2285;
- 4mS. A. Vizer, K. B. Yerzhanov, A. A. A. A. Quntar, V. M. Dembitsky, Tetrahedron 2004, 60, 5499;
- 4nI. Nakamura, Y. Yamamoto, Chem. Rev. 2004, 104, 2127.
- 5
- 5aS. Yasuhara, M. Sasa, T. Kusakabe, H. Takayama, M. Kimura, T. Mochida, K. Kato, Angew. Chem. 2011, 123, 3998;
10.1002/ange.201008139 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3912;
- 5bT. Kusakabe, Y. Kawai, R. Shen, T. Mochida, K. Kato, Org. Biomol. Chem. 2012, 10, 3133;
- 5cT. Kusakabe, E. Sekiyama, S. Motodate, S. Kato, T. Mochida, K. Kato, Synthesis 2012, 1825;
- 5dT. Kusakabe, K. Kawaguchi, M. Kawamura, N. Niimura, R. Shen, H. Takayama, K. Kato, Molecules 2012, 17, 9220;
- 5eCCC-coupling reaction of allenyl ketones: K. Kato, T. Mochida, H. Takayama, M. Kimura, H. Moriyama, A. Takeshita, Y. Kanno, Y. Inoue, H. Akita, Tetrahedron Lett. 2009, 50, 4744.
- 6
- 6aK. Kato, R. Teraguchi, S. Yamamura, T. Mochida, H. Akita, T. A. Peganova, N. V. Vologdin, O. V. Gusev, Synlett 2007, 638;
- 6bK. Kato, R. Teraguchi, S. Motodate, A. Uchida, T. Mochida, T. A. Peganova, N. V. Vologdin, H. Akita, Chem. Commun. 2008, 3687;
- 6cK. Kato, S. Motodate, T. Mochida, T. Kobayashi, H. Akita, Angew. Chem. 2009, 121, 3376; Angew. Chem. Int. Ed. 2009, 48, 3326;
- 6dS. Motodate, T. Kobayashi, M. Fujii, T. Mochida, T. Kusakabe, S. Katoh, H. Akita, K. Kato, Chem. Asian J. 2010, 5, 2221.
- 7CCDC 794263 (3), 794264 (2 n), and 888988 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See the Supporting Information.
- 8The cyclization of propargyl carbamates by intramolecular addition of a nitrogen atom to an acetylenic triple bond:
- 8aD. R. Cassady, N. R. Easton, J. Org. Chem. 1964, 29, 2032;
- 8bM. Kimura, S. Kure, Z. Yoshida, S. Tanaka, K. Fugami, Y. Tamaru, Tetrahedron Lett. 1990, 31, 4887;
- 8cK. Ohe, T. Ishihara, N. Chatani, Y. Kawasaki, S. Murai, J. Org. Chem. 1991, 56, 2267;
- 8dA. Arcadi, Synlett 1997, 941;
- 8eA. Lei, X. Lu, Org. Lett. 2000, 2, 2699.
- 9Typical experiment: A 30 mL two-neck round-bottom flask containing a magnetic stirring bar, [Pd(tfa)2(±)-L1] (9.3 mg, 0.014 mmol), p-benzoquinone (46 mg, 0.42 mmol) and MeOH (4 mL) was fitted with a rubber septum and a three-way stopcock connected to a balloon filled with carbon monoxide. The apparatus was purged with carbon monoxide by pump-filling through the three-way stopcock. A MeOH solution (1 mL) of substrate 1 n (100 mg, 0.28 mmol) was added to the stirred solution by syringe at an appropriate temperature. The remaining substrate was washed in MeOH (1 mL) twice. After stirring for 1.5 h at room temperature, the mixture was diluted with CH2Cl2 (50 mL) and washed with 3 % NaOH (40 mL). The aqueous layer was extracted with CH2Cl2 (50 mL) twice and the combined organic layers were dried over MgSO4 and concentrated in vacuo. The crude reaction mixture was purified by column chromatography on silica gel. The fraction eluted with n-hexane/ethyl acetate (1:1) afforded the spirofuranone 2 n as colorless needles.
- 10P. G. Sammes, D. J. Weller, Synthesis 1995, 1205.
- 11
- 11aI. Nitta, S. Fujimori, T. Haruyama, S. Inoue, Eur. Pat. Appl. EP 53845A1 19820616, 1982;
- 11bA. M. Turuta, T. M. Fadeeva, A. V. Kamernitskii, A. A. Korobov, E. G. Cherepanova, D. H. Luu, Izv. Akad. Nauk Ser. Khim. 1990, 3, 675.
- 12aK. P. Olive, M. A. Jacobetz, C. J. Davidson, A. Gopinathan, D. McIntyre, D. Honess, B. Madhu, M. A. Goldgraben, M. E. Caldwell, D. Allard, K. K. Frese, G. DeNicola, C. Feig, C. Combs, S. P. Winter, H. Ireland-Zecchini, S. Reichelt, W. J. Howat, A. Chang, M. Dhara, L. Wang, F. Rückert, R. Grützmann, C. Pilarsky, K. Izeradjene, S. R. Hingorani, P. Huang, S. E. Davies, W. Plunkett, M. Egorin, R. H. Hruban, N. Whitebread, K. McGovern, J. Adams, C. Iacobuzio-Donahue, J. Griffiths, D. A. Tuveson, Science 2009, 324, 1457;
- 12bT. Sakata, J. K. Chen, Chem. Soc. Rev. 2011, 40, 4318;
- 12cS. Peukert, K. Miller-Moslin, ChemMedChem 2010, 5, 500;
- 12dZ. Zhang, V. Baubet, C. Ventocilla, C. Xiang, N. Dahmane, J. D. Winkler, Org. Lett. 2011, 13, 4786;
- 12eP. Heretsch, A. Büttner, L. Tzagkaroulaki, S. Zahn, B. Kirchner, A. Giannis, Chem. Commun. 2011, 47, 7362;
- 12fC. Jin, J. P. Burgess, J. A. Kepler, C. E. Cook, Org. Lett. 2007, 9, 1887;
- 12gA. Yamaguchi, H. Ishibashi, S. Kohra, K. Arizono, K. Kato, T. Nakahama, Y. Kanno, Y. Inouye, N. Tominaga, J. Health Sci. 2009, 55, 930;
- 12hY. Kanno, R. Hikosaka, S.-Y. Zhang, Y. Inoue, T. Nakahama, K. Kato, A. Yamaguchi, N. Tominaga, S. Kohra, K. Arizono, Y. Inoue, Biol. Pharm. Bull. 2011, 34, 318.