Self-Assembling Neodymium/Sodium Heterobimetallic Asymmetric Catalyst Confined in a Carbon Nanotube Network†
Takanori Ogawa
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.htmlSearch for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
JST, ACT-C, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan)
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.htmlSearch for more papers by this authorTakanori Ogawa
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.htmlSearch for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.html
JST, ACT-C, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan)
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021 (Japan) http://www.bikaken.or.jp/research/group/shibasaki/shibasaki-lab/index_e.htmlSearch for more papers by this authorThis work was financially supported by KAKENHI (20229001 and 24106745) from JSPS and MEXT, and JST, ACT-C. N.K. thanks the Suzuken Memorial Foundation for financial support. We thank Prof. Dr. Magnus Rueping for fruitful discussions.
Graphical Abstract
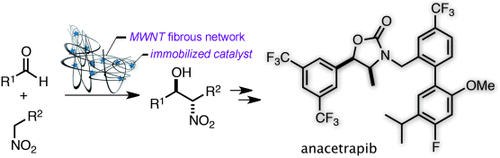
Confined cat works better: A self-assembling heterobimetallic catalyst, comprised of a Nd/Na/amide ligand confined in an entangled multiwalled carbon nanotube (MWNT) network, outperforms the unconfined catalyst in anti-selective catalytic asymmetric nitroaldol reactions. The confined catalyst could be used repeatedly through simple filtration, and was applied to a concise enantioselective synthesis of anacetrapib.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302236_sm_miscellaneous_information.pdf2.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Catalytic Asymmetric Synthesis, 2nd ed. ), Wiley-VCH, New York, 2000;
10.1002/0471721506 Google Scholar
- 1b Asymmetric Catalysis on Industrial Scale (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 1cP. J. Walsh, M. C. Kozlowski, Fundamentals of Asymmetric Catalysis, University Science Books, Sausalito, 2009;
- 1dE. J. Corey, L. Kürti, Enantioselective Chemical Synthesis, Direct Book Publishing, Dallas, 2010.
- 2For reviews on asymmetric heterogeneous catalysis, see:
- 2aJ. M. Thomas, R. Raja, D. W. Lewis, Angew. Chem. 2005, 117, 6614; Angew. Chem. Int. Ed. 2005, 44, 6456;
- 2bM. Heitbaum, F. Glorius, I. Escher, Angew. Chem. 2006, 118, 4850;
10.1002/ange.200504212 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4732;
- 2cC. Baleizão, H. Garcia, Chem. Rev. 2006, 106, 3987;
- 2dT. Mallat, E. Orglmeister, A. Baiker, Chem. Rev. 2007, 107, 4863;
- 2e Handbook of Asymmetric Heterogeneous Catalysis (Eds.: ), Wiley-VCH, Weinheim, 2008;
- 2fZ. Wang, G. Chen, K. Ding, Chem. Rev. 2009, 109, 322.
- 3
- 3a Handbook of Heterogeneous Catalysis, 2nd ed. , ), Wiley-VCH, Weinheim, 2008;
- 3b Modeling and Simulation of Heterogeneous Catalytic Reactions (Ed.: ), Wiley-VCH, Weinheim, 2011.
- 4For a review on facile non-covalent immobilization of asymmetric catalysts, see: J. M. Fraile, J. I. García, J. A. Mayoral, Chem. Rev. 2009, 109, 360.
- 5For recent examples of non-covalent immobilization of asymmetric catalysts, see:
- 5aT. Yasukawa, H. Miyamura, S. Kobayashi, J. Am. Chem. Soc. 2012, 134, 16963;
- 5bY.-X. Tan, Y.-P. He, J. Zhang, Chem. Mater. 2012, 24, 4711;
- 5cR. Jin, K. Liu, D. Xia, Q. Qian, G. Liu, H. Li, Adv. Synth. Catal. 2012, 354, 3265;
- 5dZ.-H. Li, Z.-M. Zhou, X.-Y. Hao, J. Zhang, Z. Dong, Y.-Q. Liu, Chirality 2012, 24, 1092;
- 5eY. Xu., T. Cheng, J. Long, K. Liu, Q. Qian, F. Gao, G. Liu, H. Li, Adv. Synth. Catal. 2012, 354, 3250.
- 6S. Iijima, Nature 1991, 354, 56.
- 7For reviews, see:
- 7aG. M. Whitesides, J. P. Mathias, C. T. Seto, Science 1991, 254, 1312;
- 7bJ.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, Wiley-VCH, Weinheim, 1995;
10.1002/3527607439 Google Scholar
- 7cJ.-M. Lehn, Science 2002, 295, 2400;
- 7dG. M. Whitesides, B. Grzybowski, Science 2002, 295, 2418;
- 7eR. Chakrabarty, P. Mukherjee, P. Stang, Chem. Rev. 2011, 111, 6810.
- 8
- 8aS. Akabori, S. Sakurai, Y. Izumi, Y. Fujii, Nature 1956, 178, 323;
- 8bA. Akamatsu, Y. Izumi, S. Akabori, Bull. Chem. Soc. Jpn. 1961, 35, 1706; T. Ikawa, H. Sajiki, K. Hirota, Tetrahedron 2005, 61, 2217.
- 9L. Henry, C. R. Hebd. Seances Acad. Sci. 1895, 120, 1265.
- 10For recent reviews, see:
- 10aM. Shibasaki, H. Gröger in Comprehensive Asymmetric Catalysis, Vol. III (Eds.: ), Springer, Berlin, 1999, pp. 1075–1090;
10.1007/978-3-642-58571-5_3 Google Scholar
- 10bF. A. Luzzio, Tetrahedron 2001, 57, 915;
- 10cC. Palomo, M. Oiarbide, A. Mielgo, Angew. Chem. 2004, 116, 5558;
10.1002/ange.200460506 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5442;
- 10dM. Shibasaki, H. Gröger, M. Kanai in Comprehensive Asymmetric Catalysis, Supplement 1 (Eds.: ), Springer, Heidelberg, 2004, pp. 131–133;
- 10eJ. Boruwa, N. Gogoi, P. P. Saikia, N. C. Barua, Tetrahedron: Asymmetry 2006, 17, 3315;
- 10fC. Palomo, M. Oiarbide, A. Laso, Eur. J. Org. Chem. 2007, 2561;
- 10gS. E. Milner, T. S. Moody, A. R. Maguire, Eur. J. Org. Chem. 2012, 3059.
- 11For selected examples of syn-selective catalytic asymmetric nitroaldol reactions, see:
- 11aH. Sasai, T. Tokunaga, S. Watanabe, T. Suzuki, N. Itoh, M. Shibasaki, J. Org. Chem. 1995, 60, 7388;
- 11bY. Sohtome, Y. Hashimoto, K. Nagasawa, Eur. J. Org. Chem. 2006, 2894;
- 11cT. Arai, M. Watanabe, A. Yanagisawa, Org. Lett. 2007, 9, 3595;
- 11dY. Sohtome, N. Takemura, K. Takada, R. Takagi, T. Iguchi, K. Nagasawa, Chem. Asian J. 2007, 2, 1150;
- 11eT. Arai, R. Takashita, Y. Endo, M. Watanabe, A. Yanagisawa, J. Org. Chem. 2008, 73, 4903; for partially successful examples of syn-selective catalytic asymmetric nitroaldol reactions, see:
- 11fJ. Yoshimoto, C. A. Sandoval, S. Saito, Chem. Lett. 2008, 37, 1294;
- 11gH. Y. Kim, K. Oh, Org. Lett. 2009, 11, 5682.
- 12For examples of anti-selective catalytic asymmetric Nitroaldol reactions, see:
- 12aD. Uraguchi, S. Sakaki, T. Ooi, J. Am. Chem. Soc. 2007, 129, 12392;
- 12bS. Handa, K. Nagawa, Y. Sohtome, S. Matsunaga, M. Shibasaki, Angew. Chem. 2008, 120, 3274;
10.1002/ange.200705617 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3230;
- 12cD. Uraguchi, S. Nakamura, T. Ooi, Angew. Chem. 2010, 122, 7724;
10.1002/ange.201004072 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7562;
- 12dK. Lang, J. Park, S. Hong, Angew. Chem. 2012, 124, 1652; Angew. Chem. Int. Ed. 2012, 51, 1620;
- 12eK. Xu, G. Lai, Z. Zha, S. Pan, H. Chen, Z. Wang, Chem. Eur. J. 2012, 18, 12357.
- 13For partially successful examples of anti-selective catalytic asymmetric nitroaldol reaction, see:
- 13aG. Blay, L. R. Domingo, V. Hernández-Olmos, J. R. Pedro, Chem. Eur. J. 2008, 14, 4725;
- 13bH. Ube, M. Terada, Bioorg. Med. Chem. Lett. 2009, 19, 3895;
- 13cA. Noole, K. Lippur, A. Metsala, M. Lopp, T. Kanger, J. Org. Chem. 2010, 75, 1313;
- 13dG. Blay, V. Hernández-Olmos, J. R. Pedro, Org. Lett. 2010, 12, 3058;
- 13eL. Yao, Y. Wei, P. Wang, W. He, S. Zhang, Tetrahedron 2012, 68, 9119; for anti-selective nitroaldol reaction of benzaldehyde catalyzed by hydroxynitrile lyase, see:
- 13fT. Purkarthofer, K. Gruber, M. Gruber-Khadjawi, K. Waich, W. Skranc, D. Mink, H. Griengl, Angew. Chem. 2006, 118, 3532;
10.1002/ange.200504230 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3454;
- 13gM. Gruber-Khadjawi, T. Purkarthofer, W. Skranc, H. Griengl, Adv. Synth. Catal. 2007, 349, 1445.
- 14
- 14aT. Nitabaru, N. Kumagai, M. Shibasaki, Tetrahedron Lett. 2008, 49, 272;
- 14bT. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 13860.
- 15T. Nitabaru, N. Kumagai, M. Shibasaki, Angew. Chem. 2012, 124, 1676;
10.1002/ange.201108153 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1644.
- 16The finely powdered catalyst is tiny in particle size and hardly collected by normal sintered-glass filters. Complete separation of the catalyst at low temperature is essential to obtain the product with high stereoselectivity, because raising the temperature in the presence of the catalyst significantly promotes a retro-nitroaldol reaction, diminishing the stereoselectivity.
- 17
- 17aJ. M. Planeix et al., J. Am. Chem. Soc. 1994, 116, 7935;
- 17bG. L. Bezemer, U. Falke, A. J. van Dillen, K. P. de Jong, Chem. Commun. 2005, 731;
- 17cP. Serp, M. Corrias, P. Kalck, Appl. Catal. A 2003, 253, 337;
- 17dX. Pan, X. Bao, Chem. Commun. 2008, 6271.
- 18Z. Chen, Z. Guan, M. Li, Q. Yang, C. Li, Angew. Chem. 2011, 123, 5015; Angew. Chem. Int. Ed. 2011, 50, 4913.
- 19MWNTs were mechanically fractured by shaking the test tube after 3–4 runs, and small amount of the fractured MWNTs passed through the sintered glass filter, which can be ascribed to the gradual extension of reaction time. Implementation of the MWNT-confined catalyst in a continuous flow reaction system to avoid the above problem is currently underway.
- 20
- 20aA. R. Tall, X. Jiang, Y. Luo, D. Silver, Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1185;
- 20bA. Thompson, E. Di Angelantonio, N. Sarwar, S. Erqou, D. Saleheen, R. P. Dullaart, B. Keavney, Z. Ye, J. Danesh, JAMA J. Am. Med. Assoc. 2008, 299, 2777.
- 21
- 21aC. J. Smith, A. Ali, M. L. Hammond, H. Li, Z. Lu, J. Napolitano, G. E. Taylor, C. F. Thompson, M. S. Anderson, Y. Chen, S. S. Eveland, Q. Guo, S. A. Hyland, D. P. Milot, C. P. Sparrow, S. D. Wright, A.-M. Cumiskey, M. Latham, L. B. Peterson, R. Rosa, J. V. Pivnichny, X. Tong, S. S. Xu, P. J. Sinclair, J. Med. Chem. 2011, 54, 4880;
- 21bD. E. Gutstein, R. Krishna, D. Johns, H. K. Surks, H. M. Dansky, S. Shah, Y. B. Mitchel, J. Arena, J. A. Wagner, Clin. Pharmacol. Ther. 2012, 91, 109.
- 22H. Morimoto, T. Tsubogo, N. D. Litvinas, J. F. Hartwig, Angew. Chem. 2011, 123, 3877;
10.1002/ange.201100633 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3793.
- 23S. G. Ouellet, A. Roy, C. Molinaro, R. Angelaud, J.-F. Marcoux, P. D. O’Shea, I. W. Davies, J. Org. Chem. 2011, 76, 1436.